Gut Microbiota-Derived Butyric Acid Alleviates Glucocorticoid-Associated Osteonecrosis of the Femoral Head via Modulating Inflammatory Cytokines in Bone Marrow Mesenchymal Stem Cells
- PMID: 40510588
- PMCID: PMC12162157
- DOI: 10.1155/mi/8742817
Gut Microbiota-Derived Butyric Acid Alleviates Glucocorticoid-Associated Osteonecrosis of the Femoral Head via Modulating Inflammatory Cytokines in Bone Marrow Mesenchymal Stem Cells
Abstract
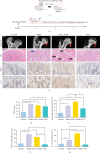
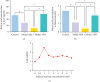
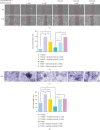
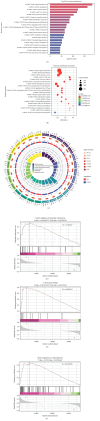
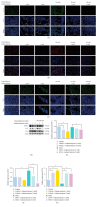
Background: The role of gut microbiota and its metabolites in regulating bone metabolism has been well established, with inflammatory immune responses potentially playing a critical role. Glucocorticoid-associated osteonecrosis of the femoral head (GA-ONFH), caused by high-dose glucocorticoid use for inflammatory or immune-related diseases, is a prevalent condition of bone metabolic imbalance. However, the regulatory role and mechanisms of gut microbiota and its metabolites in the development and progression of GA-ONFH remain unclear. This study aims to investigate the intervention effects of gut microbiota and its metabolite butyric acid on GA-ONFH through a series of multi-omics in vitro and in vivo experiments. Methods: Sprague Dawley rats were randomly divided into four groups. The gut microbial composition of the groups was analyzed through 16S rDNA sequencing. Targeted metabolomics was employed to assess differences in short-chain fatty acids (SCFAs) among the groups. Butyric acid, identified as a key differential metabolite, was then selected for further exploration of its effects on bone marrow mesenchymal stem cells (BMSCs) and GA-ONFH rat models through in vitro and in vivo experiments. Results: 16S rDNA sequencing revealed alterations in gut microbiota structure in GA-ONFH rats. Micro-CT and HE staining demonstrated that depletion of gut microbiota with broad-spectrum antibiotics prior to GA-ONFH modeling exacerbated the disease's development. In contrast, fecal microbiota transplantation (FMT) was shown to alleviate GA-ONFH progression. Targeted metabolomics indicated that FMT mitigated the reduction in butyric acid levels induced by dexamethasone (DXM). Subsequent in vitro cell experiments confirmed that butyric acid promotes BMSC proliferation, migration, and osteogenic differentiation. RNA sequencing revealed that butyric acid regulates T cell-mediated inflammatory cytokine genes in BMSCs, while Western blot and immunofluorescence assays confirmed that butyric acid modulates the expression of TNF-α and IL-2/IL-4 in BMSCs. Finally, in vivo experiments demonstrated that butyric acid supplementation attenuated the progression of GA-ONFH and improved the expression of inflammation-related cytokines in femoral head tissue. Conclusions: Our study demonstrates that gut microbiota depletion exacerbates GA-ONFH, while FMT restores butyric acid levels and alleviates disease severity. Butyric acid reduced the expression of TNF-α and IL-2 while increasing the level of IL-4 in vivo and in vitro, thereby improving the local inflammatory environment of the femoral head and alleviating the progression of GA-ONFH. These findings highlight that reduction in butyric acid levels due to gut microbiota dysbiosis is a crucial factor in the progression of GA-ONFH.
Keywords: BMSCs; GA-ONFH; bone metabolism; butyrate acid; gut microbiota.
Copyright © 2025 Shuai He et al. Mediators of Inflammation published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
[Yougui Yin attenuates adipogenic differentiation of bone marrow mesenchymal stem cells by modulating PPARγ pathway to treat glucocorticoid-induced osteonecrosis].Zhongguo Zhong Yao Za Zhi. 2025 Jun;50(12):3356-3367. doi: 10.19540/j.cnki.cjcmm.20250307.401. Zhongguo Zhong Yao Za Zhi. 2025. PMID: 40686113 Chinese.
-
MiR-370-3p regulate TLR4/SLC7A11/GPX4 to alleviate the progression of glucocorticoids-induced osteonecrosis of the femoral head by promoting osteogenesis and suppressing ferroptosis.J Orthop Translat. 2025 Feb 12;51:337-358. doi: 10.1016/j.jot.2024.10.014. eCollection 2025 Mar. J Orthop Translat. 2025. PMID: 40584015 Free PMC article.
-
Multi-omics analyses reveal that hesperidin ameliorates high-altitude pulmonary hypertension by restoring gut-lung axis homeostasis.Phytomedicine. 2025 Sep;145:157069. doi: 10.1016/j.phymed.2025.157069. Epub 2025 Jul 11. Phytomedicine. 2025. PMID: 40680331
-
Role of the intestinal flora-immunity axis in the pathogenesis of rheumatoid arthritis-mechanisms regulating short-chain fatty acids and Th17/Treg homeostasis.Mol Biol Rep. 2025 Jun 21;52(1):617. doi: 10.1007/s11033-025-10714-w. Mol Biol Rep. 2025. PMID: 40544212 Review.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

