CRISPR antiviral inhibits neurotrophic JC polyomavirus in 2D and 3D culture models through dual-gRNA excision by SaCas9
- PMID: 40510594
- PMCID: PMC12159223
- DOI: 10.1016/j.omtn.2025.102556
CRISPR antiviral inhibits neurotrophic JC polyomavirus in 2D and 3D culture models through dual-gRNA excision by SaCas9
Abstract
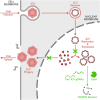
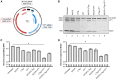
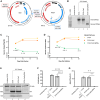
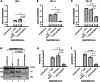
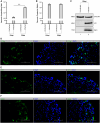
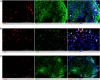
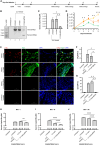
Without an effective antiviral, JC virus (JCV) has persisted throughout multiple epochs of immunosuppression, causing the opportunistic demyelinating disease, progressive multifocal leukoencephalopathy (PML). This study proposes a novel therapy using a dual-gRNA, SaCas9, CRISPR antiviral targeting JCV transcription factor, large tumor antigen (LT-Ag), and capsid protein, viral protein 1 (VP1). This treatment was validated using traditional two-dimensional cell culture. A recombinant cell line was produced from SVG astrocytes (SVGA) via lentiviral inoculation and puromycin selection. Following infection, sanger sequencing identified uniform excision of the circular dsDNA genome of JCV, significantly reducing viral load per genomic copy number on qPCR, viral proteins on western blot, and infectivity of viral progeny on adoptive transfer. Following this proof-of-concept using cell lines, translatability of results was advanced using three-dimensional, heterogeneous cerebral organoids (COs). COs were infected and treated with the lentivirus-packaged CRISPR antiviral. As observed in monolayer culture, a truncated genome was confirmed with sequencing, reducing viral load per genomic copy number on qPCR, protein levels on immunofluorescent imaging, and infectivity on adoptive transfer. The high efficacy of this JCV-targeting CRISPR antiviral in the context of cerebral organoids expounds on its value for the currently untreatable JCV and PML.
Keywords: CRISPR; JC virus; MT: RNA/DNA Editing; antiviral; cerebral organoids; gene editing; progressive multifocal leukoencephalopathy.
© 2025 The Authors.
Conflict of interest statement
A.R., S.L., C.C., H.L., S.C., A.B., I.K.S., and H.S.W. declare no conflict of interest. K.K. is named inventor on patents that cover the viral gene editing technology that is the subject of this article. K.K. is a co-founder, board member, scientific advisor, and holds equity in Excision BioTherapeutics, a biotech startup that has licensed the viral gene editing technology from Temple University for commercial development and clinical trials.
Figures
Similar articles
-
Synthetic antibodies and peptides recognizing progressive multifocal leukoencephalopathy-specific point mutations in polyomavirus JC capsid viral protein 1.MAbs. 2015;7(4):681-92. doi: 10.1080/19420862.2015.1038447. Epub 2015 Apr 16. MAbs. 2015. PMID: 25879139 Free PMC article.
-
CRISPR/Cas9 System as an Agent for Eliminating Polyomavirus JC Infection.PLoS One. 2015 Sep 11;10(9):e0136046. doi: 10.1371/journal.pone.0136046. eCollection 2015. PLoS One. 2015. PMID: 26360417 Free PMC article.
-
Deep-Sequence Identification and Role in Virus Replication of a JC Virus Quasispecies in Patients with Progressive Multifocal Leukoencephalopathy.J Virol. 2016 Dec 16;91(1):e01335-16. doi: 10.1128/JVI.01335-16. Print 2017 Jan 1. J Virol. 2016. PMID: 27795410 Free PMC article.
-
Overview of the cellular immunity against JC virus in progressive multifocal leukoencephalopathy.J Neurovirol. 2002 Dec;8 Suppl 2:59-65. doi: 10.1080/13550280290167894. J Neurovirol. 2002. PMID: 12491153 Review.
-
The importance of mouse models to define immunovirologic determinants of progressive multifocal leukoencephalopathy.Front Immunol. 2015 Jan 5;5:646. doi: 10.3389/fimmu.2014.00646. eCollection 2014. Front Immunol. 2015. PMID: 25601860 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials

