Engineering 3D-BMSC exosome-based hydrogels that collaboratively regulate bone microenvironment and promote osteogenesis for enhanced cell-free bone regeneration
- PMID: 40510837
- PMCID: PMC12159235
- DOI: 10.1016/j.mtbio.2025.101881
Engineering 3D-BMSC exosome-based hydrogels that collaboratively regulate bone microenvironment and promote osteogenesis for enhanced cell-free bone regeneration
Abstract
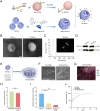
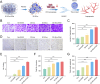
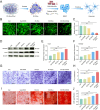
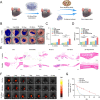
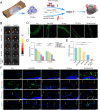
Large bone defects are a significant clinical challenge due to their frequent failure to heal spontaneously. Recently, BMSC-derived exosomes (Exo) based cell-free bone regeneration offer several distinct advantages over BMSCs themselves in the repair of damaged tissue, including enhanced repair ability and superior biocompatibility, which can be further augmented under 3D-cultured conditions. However, their therapeutic efficacy for bone regeneration is significantly constrained by hypoxic bone microenvironment and short retention time in bone defect region. Thus, judiciously regulating bone microenvironment and extending retention time are crucial for bone regeneration. Herein, we developed Superparamagnetic Iron Oxide Nanoparticles (SPION) -modified 3D-cultured Exo, termed as 3D-SExo, to enhance Reactive Oxygen Species (ROS) scavenging and promote bone regeneration in response to the needs of bone defect. After entrapment in bone-targeting peptide-modified GelMA (Gel-DSS6), the composite hydrogel (3D-SExo/DGel) was obtained, which can prolong the retention of exosomes, and thereby enhancing bone repair ability. In addition, miR-122-5p, detected via microRNA (miRNA) array from 3D-cultured Exo, were observed to promote osteogenesis by activating Wnt/β-catenin pathway, which was further verified by miRNA transfection. Through the in vitro and in vivo studies, 3D-SExo/DGel could decompose ROS to relive hypoxia and alleviate the inhibitory effect of ROS on β-catenin production, demonstrating significant clinical therapeutic potential to improve cell-free bone regeneration.
Keywords: Bone-targeted hydrogels; Collaboratively promote osteogenesis; Engineered 3D-BMSC Exosome; Enhanced cell‐free bone regeneration; Reactive oxygen species; Wnt/β-catenin pathway.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



Similar articles
-
Lithium Promotes Osteogenesis via Rab11a-Facilitated Exosomal Wnt10a Secretion and β-Catenin Signaling Activation.ACS Appl Mater Interfaces. 2024 Jun 19;16(24):30793-30809. doi: 10.1021/acsami.4c04199. Epub 2024 Jun 4. ACS Appl Mater Interfaces. 2024. PMID: 38833412
-
Bone microenvironment regulative hydrogels with ROS scavenging and prolonged oxygen-generating for enhancing bone repair.Bioact Mater. 2023 Jan 9;24:477-496. doi: 10.1016/j.bioactmat.2022.12.021. eCollection 2023 Jun. Bioact Mater. 2023. PMID: 36714330 Free PMC article.
-
Optimized BMSC-derived osteoinductive exosomes immobilized in hierarchical scaffold via lyophilization for bone repair through Bmpr2/Acvr2b competitive receptor-activated Smad pathway.Biomaterials. 2021 May;272:120718. doi: 10.1016/j.biomaterials.2021.120718. Epub 2021 Mar 27. Biomaterials. 2021. PMID: 33838528
-
Extracellular vesicle-loaded hydrogels for tissue repair and regeneration.Mater Today Bio. 2022 Dec 21;18:100522. doi: 10.1016/j.mtbio.2022.100522. eCollection 2023 Feb. Mater Today Bio. 2022. PMID: 36593913 Free PMC article. Review.
-
Exosome-Laden Hydrogels: A Novel Cell-free Strategy for In-situ Bone Tissue Regeneration.Front Bioeng Biotechnol. 2022 Apr 1;10:866208. doi: 10.3389/fbioe.2022.866208. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35433664 Free PMC article. Review.
References
-
- Tarchala M., Harvey E.J., Barralet J. Biomaterial-stabilized soft tissue healing for healing of critical-sized bone defects: the masquelet technique. Adv. Healthc. Mater. 2016;5(6):630–640. - PubMed
-
- Quarto R., Mastrogiacomo M., Cancedda R., Kutepov S.M., Mukhachev V., Lavroukov A., Kon E., Marcacci M. Repair of large bone defects with the use of autologous bone marrow stromal cells, TN. Engl. J. Med. 2001;344(5):385–386. - PubMed
-
- Wang Y., Wang J., Gao R., Liu X., Feng Z., Zhang C., Huang P., Dong A., Kong D., Wang W. Biomimetic glycopeptide hydrogel coated PCL/nHA scaffold for enhanced cranial bone regeneration via macrophage M2 polarization-induced osteo-immunomodulation. Biomaterials. 2022;285 - PubMed
LinkOut - more resources
Full Text Sources

