Infection, Relapses, and Pseudo-Relapses in Individuals With Multiple Sclerosis
- PMID: 40510870
- PMCID: PMC12153504
- DOI: 10.1212/CPJ.0000000000200493
Infection, Relapses, and Pseudo-Relapses in Individuals With Multiple Sclerosis
Abstract
Background and objectives: Infections are associated with an increased risk of relapse and pseudo-relapse in persons with multiple sclerosis (MS). However, the relationship with relapses and pseudo-relapses after SARS-CoV-2 infections (COVID) vs other infections in MS is poorly understood. Therefore, we compared the occurrence of relapse and pseudo-relapse after COVID and other infections with noninfected participants with MS.
Methods: In spring 2023, we surveyed participants from the North American Research Committee on Multiple Sclerosis Registry regarding whether they had had a COVID infection, other infections, relapses, and pseudo-relapses. Recent infections, occurring in the 6 months before the survey, were used to categorize participants into groups: recent COVID, non-COVID infection (with no history of ever having COVID), COVID and non-COVID infections, or uninfected.
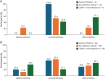
Results: Of the 4,787 participants eligible for analysis, 2,927 participants were included, of whom 294 (10%) had a recent COVID infection; 853 (29.1%) had 1 recent infection other than COVID; 246 (8.4%) had a recent COVID and non-COVID infection; and 1,534 (52.4%) had no infection with COVID nor any infection within the past 6 months (uninfected). Compared with no infections, non-COVID infection was associated with a 39% increased likelihood of relapse (1.39, 95% CI [1.04-1.87]), whereas a recent COVID infection was associated with a decreased likelihood of relapse (0.45 [0.23, 0.87]), adjusting for covariates. All infection groups were associated with increased odds of pseudo-relapse compared with the uninfected group (non-COVID infections: 1.78 [1.44, 2.20]; COVID infection: 1.80 [1.32, 2.45]; COVID and non-COVID infection: 3.04 [2.24, 4.12]).
Discussion: Because individuals with MS are at increased risk of infections, the association of infections with relapses and pseudo-relapses is clinically important. The high prevalence of acute worsening after infection, regardless of the type of infection, compared with those with no reported infection, needs to be considered in the management of persons with MS.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Conflict of interest statement
A. Salter receives research funding from the Multiple Sclerosis Society of Canada, the National Multiple Sclerosis Society, the Consortium of Multiple Sclerosis Centers (CMSC), and the Department of Defense Congressionally Directed Medical Research Program; is a member of the editorial board of Neurology®; serves as a consultant for Gryphon Bio LLC, Sora Neuroscience, and Abata Therapeutics; has equity in Owl Therapeutics; is a member of the data and safety monitoring board for Premature Infants Receiving Milking or Delayed Cord Clamping, Central Vein Sign: A Diagnostic Biomarker in Multiple Sclerosis, and Methotrexate treatment of Arthritis caused by Chikungunya virus (March); and holds the Kenney Marie Dixon‐Pickens Distinguished Professorship in Multiple Sclerosis Research. S. Lancia and M. Sharma have nothing to disclose. G. Cutter serves on data and safety monitoring boards for Applied Therapeutics, AI Therapeutics, AMO Pharma, Astra-Zeneca, Avexis Pharmaceuticals, Biolinerx, Brainstorm Cell Therapeutics, Bristol Meyers Squibb/Celgene, CSL Behring, Galmed Pharmaceuticals, Green Valley Pharma, Horizon Pharmaceuticals, Immunic, Karuna Therapeutics, Mapi Pharmaceuticals LTD, Merck, Mitsubishi Tanabe Pharma Holdings, Opko Biologics, Prothena Biosciences, Novartis, Regeneron, Sanofi-Aventis, Reata Pharmaceuticals, Teva Pharmaceuticals, National Heart, Lung, and Blood Institute (Protocol Review Committee), University of Texas Southwestern, University of Pennsylvania, and Visioneering Technologies Inc.; has served on consulting or advisory boards for Alexion, Antisense Therapeutics, Biogen, Clinical Trial Solutions LLC, Entelexo Biotherapeutics Inc., Genzyme, Genentech, GW Pharmaceuticals, Immunic, Immunosis Pty Ltd, Klein-Buendel Incorporated, Merck/Serono, Novartis, Perception Neurosciences, Protalix Biotherapeutics, Regeneron, Roche, and SAB Biotherapeutics; is employed by the University of Alabama at Birmingham; and is president of Pythagoras Inc., a private consulting company located in Birmingham, AL. R.J. Fox has received personal consulting fees from Astoria Biologica, Biogen, Bristol Myers Squibb, Cognito, EMD Serono, Galvani, Immunic, INmune Bio, Kiniksa, Novartis, Sanofi, Siemens, TG Therapeutics, and Viracta; served on advisory committees for AB Science, Biogen, Immunic, Novartis, and Sanofi; received clinical trial contract and research grant funding from Biogen, Novartis, and Sanofi; and serves on the editorial boards of Neurology® and Multiple Sclerosis Journal. R.A. Marrie receives research funding from Canadian Institutes of Health Research, MS Canada, Crohn's and Colitis Canada, the National Multiple Sclerosis Society, CMSC, the Arthritis Society, the US Department of Defense, the Pfizer Foundation, and the Public Health Agency of Canada; is a coinvestigator on studies receiving funding from Biogen Idec and Roche Canada; holds the Multiple Sclerosis Chair in Clinical Research; and serves on the editorial board of Neurology®. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.TAKE-HOME POINTS→ Infection(s) were associated with an increase in the odds of a pseudo-relapse.→ Non-COVID infections were associated with increased odds of relapse.→ Because individuals with MS are at increased risk of infections, the association of infections with relapses and pseudo-relapses is important for clinical care.
Figures


Similar articles
-
Incidence of multiple sclerosis relapses and pseudo-relapses following COVID-19 vaccination.Mult Scler Relat Disord. 2023 Sep;77:104865. doi: 10.1016/j.msard.2023.104865. Epub 2023 Jul 2. Mult Scler Relat Disord. 2023. PMID: 37418929
-
Effects of COVID-19 Infection on Symptom Severity and Disability in Multiple Sclerosis.Neurology. 2025 Jan 28;104(2):e210149. doi: 10.1212/WNL.0000000000210149. Epub 2024 Dec 23. Neurology. 2025. PMID: 39715473 Free PMC article.
-
Post-acute sequela of COVID-19 infection in individuals with multiple sclerosis.Mult Scler. 2025 Mar;31(3):314-323. doi: 10.1177/13524585241310104. Epub 2025 Jan 3. Mult Scler. 2025. PMID: 39749575 Free PMC article.
-
COVID-19 and the risk of CNS demyelinating diseases: A systematic review.Front Neurol. 2022 Sep 20;13:970383. doi: 10.3389/fneur.2022.970383. eCollection 2022. Front Neurol. 2022. PMID: 36203986 Free PMC article.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
References
-
- Klineova S, Harel A, Straus Farber R, et al. EOutcomes of COVID-19 infection in multiple sclerosis and related conditions: one-year pandemic experience of the multicenter New York COVID-19 Neuroimmunology Consortium (NYCNIC). Mult Scler Relat Disord. 2021;55:103153. doi: 10.1016/j.msard.2021.103153 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
