Gallbladder Mixed Neuroendocrine-Non-Neuroendocrine Neoplasm Consisting of Adenocarcinoma and Neuroendocrine Tumor G2 Diagnosed after Surgery for Acute Cholecystitis: A Case Report and Exome Analysis
- PMID: 40511448
- PMCID: PMC12162149
- DOI: 10.70352/scrj.cr.24-0097
Gallbladder Mixed Neuroendocrine-Non-Neuroendocrine Neoplasm Consisting of Adenocarcinoma and Neuroendocrine Tumor G2 Diagnosed after Surgery for Acute Cholecystitis: A Case Report and Exome Analysis
Abstract
Introduction: Among neuroendocrine neoplasms (NENs), non-neuroendocrine and NEN components may rarely coexist, which are referred to as mixed neuroendocrine-non-NENs (MiNENs). Most gallbladder MiNENs are progressive and associated with neuroendocrine carcinoma (NEC), but rarely with neuroendocrine tumor (NET) as a component. To our knowledge, there are 4 reported cases of mixed gallbladder tumors with NET as a component. From the genetic analysis of MiNENs consisting of NEC, MiNEN is believed to have a common origin, as each tumor component shares a common TP53 mutation. Our case is an extremely rare reported case of a mixed gallbladder tumor with a NET component as a MiNEN, and the first reported case of whole-exome analysis performed on a resected specimen.
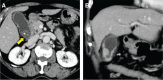
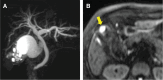
Case presentation: A 77-year-old woman presented to our hospital with epigastric pain. An emergency laparoscopic cholecystectomy was performed with a diagnosis of acute gallstone cholecystitis. Pathological examination revealed gallbladder MiNEN (adenocarcinoma + NET G2). Additional surgery was performed, but no residual tumor was found. The patient has been recurrence-free for 36 months after surgery without adjuvant therapy. The origin of the tumor was examined. Macroscopically, adenocarcinoma cells were present on both sides of the NET, while microscopically, some adenocarcinoma cells were positive for neuroendocrine markers (synaptophysin and chromogranin A). Staining for p53 showed wild-type staining with scattered, weakly expressing cells in both tumors. Subsequently, we performed whole-exome sequencing of each tumor component. The results showed that each tumor component shared TP53 c.1015G>T (p.Glu339Ter), ERBB3 c.889G>A (p.Asp297Asn), and CDKN2A c.416G>A (p.Gly139Asp) mutations, suggesting that the adenocarcinoma might have differentiated into NET G2.
Conclusions: In this case report, the tumors shared a common genetic mutation, suggesting that MiNENs with NET components may share a common origin. Furthermore, the NEN component of MiNEN occurring in the gallbladder was associated with a TP53 mutation, despite the low frequency of TP53 mutations in normal NETs.
Keywords: acute cholecystitis; gallbladder cancer; mixed neuroendocrine–non-neuroendocrine neoplasms (MiNENs).
© 2025 The Author(s). Published by Japan Surgical Society.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
Gallbladder Mixed Neuroendocrine-Non-neuroendocrine Neoplasm (MiNEN) Arising in Intracholecystic Papillary Neoplasm: Clinicopathologic and Molecular Analysis of a Case and Review of the Literature.Endocr Pathol. 2020 Mar;31(1):84-93. doi: 10.1007/s12022-020-09605-6. Endocr Pathol. 2020. PMID: 31981075 Review.
-
Mixed Intraepithelial Neoplasia-Well-Differentiated Neuroendocrine Tumor of the Gallbladder: A Hitherto Unreported Combination.Int J Surg Pathol. 2021 Sep;29(6):685-689. doi: 10.1177/1066896920986987. Epub 2021 Jan 9. Int J Surg Pathol. 2021. PMID: 33423570
-
Mixed neuroendocrine-non-neuroendocrine neoplasms (MiNEN) in the non-ampullary region of the duodenum: a case report.J Surg Case Rep. 2025 Apr 5;2025(4):rjaf189. doi: 10.1093/jscr/rjaf189. eCollection 2025 Apr. J Surg Case Rep. 2025. PMID: 40191662 Free PMC article.
-
[Analysis of clinicopathological characteristics, therapeutic strategy and prognosis of 501 patients with gastric neuroendocrine neoplasms attending a single center].Zhonghua Wei Chang Wai Ke Za Zhi. 2023 May 25;26(5):459-466. doi: 10.3760/cma.j.cn441530-20220512-00212. Zhonghua Wei Chang Wai Ke Za Zhi. 2023. PMID: 37217354 Chinese.
-
Mixed neuroendocrine-non-neuroendocrine neoplasm of the gallbladder: case report and literature review.Diagn Pathol. 2022 Jun 17;17(1):51. doi: 10.1186/s13000-022-01231-6. Diagn Pathol. 2022. PMID: 35715834 Free PMC article. Review.
References
-
- Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008; 26: 3063–72. - PubMed
-
- Klimstra DS, Klöppel G, La Rosa S, et al. Classification of neuroendocrine neoplasms of the digestive system. In: WHO Classification of Tumours Editorial Board, ed. World Health Organ Classification of Tumours. 5th ed. Digestive System Tumours. Lyon: The International Agency for Research on Cancer (IARC); 2019. p.16–9.
-
- Harada K, Sato Y, Ikeda H, et al. Clinicopathologic study of mixed adenoneuroendocrine carcinomas of hepatobiliary organs. Virchows Arch 2012; 460: 281–9. - PubMed
-
- Shintaku M, Kataoka K, Kawabata K. Mixed adenoneuroendocrine carcinoma of the gallbladder with squamous cell carcinomatous and osteosarcomatous differentiation: report of a case. Pathol Int 2013; 63: 113–9. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

