Influenza vaccine effectiveness in Europe and the birth cohort effect against influenza A(H1N1)pdm09: VEBIS primary care multicentre study, 2023/24
- PMID: 40511473
- PMCID: PMC12164280
- DOI: 10.2807/1560-7917.ES.2025.30.23.2500011
Influenza vaccine effectiveness in Europe and the birth cohort effect against influenza A(H1N1)pdm09: VEBIS primary care multicentre study, 2023/24
Abstract
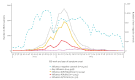
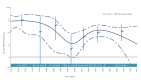
IntroductionInfluenza A(H1N1)pdm09, A(H3N2) and B/Victoria viruses circulated in Europe in 2023/24, with A(H1N1)pdm09 dominance. First influenza infections in childhood may lead to different vaccine effectiveness (VE) in subsequent years.AimThe VEBIS primary care network estimated influenza VE in Europe using a multicentre test-negative study.MethodsPrimary care practitioners collected information and specimens from patients consulting with acute respiratory infection. We estimated VE against influenza (sub)type and clade, by age group and by year of age for A(H1N1)pdm09, using logistic regression.ResultsWe included 29,958 patients, with 3,054, 1,053 and 311 influenza A(H1N1)pdm09, A(H3N2) and B cases, respectively. All-age VE against influenza A(H1N1)pdm09 was 52% (95% CI: 44-59). By year of age, VE was 27% (95% CI: -2 to 47) at 44 years with peaks at 72% (95% CI: 52-84) and 54% (95% CI: 41-64) among children and those 65 years and older, respectively. All-age A(H1N1)pdm09 VE against clade 5a.2a was 41% (95% CI: 24-54) and -11% (95% CI: -69 to 26) against clade 5a.2a.1. The A(H3N2) VE was 35% (95% CI: 20-48) among all ages and ranged between 34% and 40% by age group. All-age VE against clade 2a.3a.1 was 38% (95% CI: 1-62). All-age VE against B/Victoria was 83% (95% CI: 65-94), ranging between 70 and 92% by age group.DiscussionThe 2023/24 VEBIS primary care VE against medically attended symptomatic influenza infection was high against influenza B/Victoria, but lower against influenza A(H1N1)pdm09 and A(H3N2). Clade- and age-specific effects may have played a role in the lower A(H1N1)pdm09 VE.
Keywords: Influenza; case-control study; imprinting; influenza vaccines; multicentre study; vaccine effectiveness.
Conflict of interest statement
Figures

References
-
- World Health Organization (WHO) Recommended composition of influenza virus vaccines for use in the 2023-2024 northern hemisphere influenza season. Geneva: WHO; 2023. Available from: https://www.who.int/publications/m/item/recommended-composition-of-influ...
-
- European Centre for Disease Prevention and Control (ECDC). European Respiratory Virus Surveillance Summary (ERVISS). Stockholm: ECDC. [Accessed: 25 Jun 2024]. Available from: https://erviss.org
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
