Hederagenin promotes SIRT6 to attenuate epidural scar formation by aggravating PRMT1 deacetylation
- PMID: 40511498
- PMCID: PMC12163784
- DOI: 10.1302/2046-3758.146.BJR-2024-0287.R2
Hederagenin promotes SIRT6 to attenuate epidural scar formation by aggravating PRMT1 deacetylation
Abstract
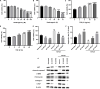
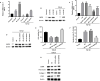
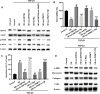
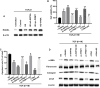
Aims: The formation of a postoperative epidural scar induced by epidural fibrosis is the main reason for recurrence of lumbar disc herniation after laminectomy. Hederagenin (HE) has been found to be widely present in various medicinal plants and has various pharmacological functions. This study aimed to investigate the effect and regulatory mechanism of HE on epidural scar formation.
Methods: Transforming growth factor beta 1 (TGF-β1)-stimulated epidural scar fibroblasts were used as an in vitro cell model. Based on that, HE treatment was carried out along with sirtuin-6 (SIRT6) silence or protein arginine N-methyltransferase 1 (PRMT1) overexpression. The interaction between SIRT6 and PRMT1 was evaluated by pulldown and co-immunoprecipitation (CoIP) assays. Then, cell proliferation, apoptosis, and fibrosis were measured by Cell Counting Kit (CCK)-8, flow cytometry, and western blotting. Moreover, the effects of receptor activator of nuclear factor-κB ligand (RANKL) supplementation and endoplasmic reticulum (ER) stress were also evaluated by supplementing recombinant protein and specific inhibitor or activator.
Results: HE depressed cell proliferation and fibrosis, while inducing apoptosis of epidural fibroblasts. Meanwhile, HE promoted SIRT6 expression which suppressed PRMT1 acetylation and protein stability. Additionally, HE induced ER stress and upregulated RANKL in epidural fibroblasts via mediating SIRT6/PRMT1 axis.
Conclusion: Generally, the therapeutic role of HE treatment on epidural scar formation was exerted by regulating SIRT6/PRMT1 axis-mediated ER stress and RANKL pathway. This study provides evidence of a novel therapeutic measure for epidural scar formation.
© 2025 Fan and Wang.
Conflict of interest statement
J. Wang declares a grant from the National Natural Science Foundation of China for this study. X-C. Fan reports grants from the Key Research and Development Program of Shaanxi (grant numbers: 2022ZDLSF04-10, 2023-YBSF-617).
Figures

Similar articles
-
The role and mechanisms of action of SIRT6 in the suppression of postoperative epidural scar formation.Int J Mol Med. 2016 May;37(5):1337-44. doi: 10.3892/ijmm.2016.2522. Epub 2016 Mar 11. Int J Mol Med. 2016. PMID: 26987016
-
PRMT1 Upregulates SIRT6 by Enhancing Arginine Methylation of E2F7 to Inhibit Vascular Smooth Muscle Cell Senescence in Aortic Dissection.FASEB J. 2025 May 15;39(9):e70579. doi: 10.1096/fj.202403269R. FASEB J. 2025. PMID: 40298071
-
Negative regulation of PI3K/AKT/mTOR axis regulates fibroblast proliferation, apoptosis and autophagy play a vital role in triptolide-induced epidural fibrosis reduction.Eur J Pharmacol. 2019 Dec 1;864:172724. doi: 10.1016/j.ejphar.2019.172724. Epub 2019 Oct 7. Eur J Pharmacol. 2019. PMID: 31600493
-
Pirfenidone inhibits epidural scar fibroblast proliferation and differentiation by regulating TGF-β1-induced Smad-dependent and -independent pathways.Am J Transl Res. 2019 Mar 15;11(3):1593-1604. eCollection 2019. Am J Transl Res. 2019. PMID: 30972185 Free PMC article.
-
Potential roles of lncRNA-Cox2 and EGR1 in regulating epidural fibrosis following laminectomy.Eur Rev Med Pharmacol Sci. 2019 Sep;23(17):7191-7199. doi: 10.26355/eurrev_201909_18820. Eur Rev Med Pharmacol Sci. 2019. PMID: 31539105
References
Grants and funding
LinkOut - more resources
Full Text Sources

