Proteogenomic and observational evidence implicate ANGPTL4 as a potential therapeutic target for colorectal cancer prevention
- PMID: 40511612
- PMCID: PMC12415964
- DOI: 10.1093/jnci/djaf137
Proteogenomic and observational evidence implicate ANGPTL4 as a potential therapeutic target for colorectal cancer prevention
Abstract
Background: The role of lipid-perturbing medications in cancer risk is unclear.
Methods: We employed cis-Mendelian randomization and colocalization to evaluate the role of 5 lipid-perturbing drug targets (ANGPTL3, ANGPTL4, APOC3, CETP, and PCSK9) in risk of 5 cancers (breast, colorectal, head and neck, ovarian, and prostate). We triangulated findings using pre-diagnostic protein measures in prospective analyses in EPIC (977 colorectal cancer cases, 4080 sub-cohort members) and the UK Biobank (860 colorectal cancer cases, 50 177 controls). To gain mechanistic insight into the role of ANGPTL4 in carcinogenesis, we examined the impact of the ANGPTL4 p. E40K loss-of-function variant on differential gene expression in normal colon tissue in BarcUVa-Seq. Finally, we evaluated the association of colon tumor ANGPTL4 expression with cancer-specific mortality in TCGA.
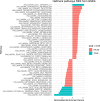
Results: In analysis of 78 473 cases and 107 143 controls, genetically proxied circulating ANGPTL4 inhibition was associated with reduced colorectal cancer risk (ORSD decrease = 0.76, 95% confidence interval [CI] = 0.66 to 0.89, P = 5.52 × 10-4, PPcolocalization = 0.83). This association was replicated using pre-diagnostic circulating ANGPTL4 concentrations in EPIC (hazard ratio [HR]log10 decrease = 0.91, 95% CI = 0.84 to 0.98, P = .01) and the UK Biobank (HRSD decrease = 0.93, 95% CI = 0.86 to 0.99, P = .03). In gene-set enrichment analysis of differential gene expression in 445 colon tissue samples, ANGPTL4 loss-of-function down-regulated several cancer-related biological pathways (PFDR < .05), including those involved in cellular proliferation, epithelial-to-mesenchymal transition, and bile acid metabolism. In analysis of 465 colon cancer patients, lower ANGPTL4 tumor expression was associated with reduced colorectal cancer-specific mortality risk (HRlog2 decrease = 0.66, 95% CI = 0.50 to 0.87, P = 2.92 × 10-3).
Conclusions: Our integrative proteogenomic and observational analyses suggest a potential protective role of lower circulating ANGPTL4 concentrations in colorectal cancer risk. These findings support further evaluation of ANGPTL4 as a therapeutic target for colorectal cancer prevention.
© The Author(s) 2025. Published by Oxford University Press.
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures

References
MeSH terms
Substances
Grants and funding
- 001/WHO_/World Health Organization/International
- National Institute for Health and Care Research Imperial Biomedical Research Centre
- APR7_1002/A*STAR (UIBR), the Academy of Medical Sciences Professorship
- EP/V029045/1/Engineering and Physical Sciences Research Council
- GCTRA18022MORE/Spanish Association Against Cancer
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

