Monoclonal antibody neutralizes Staphylococcus aureus serine protease-like protein B (SplB)-induced pathology
- PMID: 40512005
- PMCID: PMC12234435
- DOI: 10.1128/iai.00171-25
Monoclonal antibody neutralizes Staphylococcus aureus serine protease-like protein B (SplB)-induced pathology
Abstract
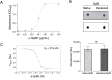
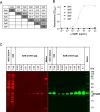
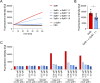
Staphylococcus aureus is a versatile pathogen, renowned for its arsenal of virulence and immune evasion factors. Several S. aureus virulence factors have been targeted in vaccination trials; however, so far, without success. Promising new vaccine candidates are the staphylococcal serine protease-like proteins (Spl A-F), which are involved in the pathogenesis and immune evasion of S. aureus. SplB, for instance, promotes type 2 immune responses and inactivates human complement factors. In this study, we report on the production and characterization of a murine monoclonal antibody (mAb) against SplB. The murine anti-SplB mAb α-SplB1 was produced by hybridoma technology, and its binding characteristics were investigated using enzyme-linked immunosorbent assay (ELISA), Western blot, and MicroScale Thermophoresis. Its neutralizing capacity was determined in a fluorogenic substrate assay, Western blot, and a murine vascular leakage model. α-SplB1 bound to recombinant SplB with high specificity, showing no cross-reactivity to other Spls or secreted proteins of S. aureus. MicroScale Thermophoresis revealed a KD value of 37.9 nM for the α-SplB1:SplB interaction. α-SplB1 neutralized the enzymatic activity of SplB in vitro in a dose-dependent manner, yielding complete neutralization at a twofold molar excess of the antibody. In a murine vascular leakage model, the antibody completely abolished SplB-mediated endothelial damage. In summary, we produced a neutralizing mAb against the staphylococcal protease SplB, which merits further investigation as a candidate for the immunotherapy of SplB-induced pathologies.
Keywords: antibody characterization; bacterial protease; host-pathogen interaction; neutralization; passive vaccination; vaccine candidate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- 2024. Point prevalence survey of healthcare-associated infections and antimicrobial use in European acute care hospitals - 2020-2023. European Centre for Disease Prevention and Control, Stockholm.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

