Oxygen and immunity to Leishmania infection
- PMID: 40512023
- PMCID: PMC12234431
- DOI: 10.1128/iai.00504-24
Oxygen and immunity to Leishmania infection
Abstract
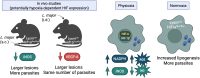
Oxygen availability plays a fundamental role in shaping immune responses to infections. Leishmaniasis, a disease caused by protozoan parasites of the genus Leishmania, manifests in a spectrum of clinical outcomes, ranging from localized cutaneous lesions to life-threatening visceral infections. Like many infections and chronic diseases, Leishmania-infected tissues are characterized by hypoxia. Despite the recognized importance of oxygen in immune regulation, our understanding of how hypoxia shapes the immune landscape in leishmaniasis remains in its early stages. Collectively, the published studies in leishmaniasis highlight the critical role of oxygen availability and hypoxia-inducible factor (HIF) in orchestrating immune responses, particularly within myeloid cells. Here, we review the literature on how oxygen availability and HIF signaling influence the immune response in leishmaniasis. By consolidating existing findings and identifying gaps in knowledge, we aim to inspire further research into the interplay between oxygen availability, immune function, disease progression, and therapeutic potential in leishmaniasis.
Keywords: hypoxia; immune responses; leishmaniasis; oxygen; parasites.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Warburg O. 1956. On the origin of cancer cells. Science. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

