Modeling potential cost-effectiveness of tirzepatide versus lifestyle modification for patients with overweight and obesity
- PMID: 40512029
- PMCID: PMC12210105
- DOI: 10.1002/oby.24310
Modeling potential cost-effectiveness of tirzepatide versus lifestyle modification for patients with overweight and obesity
Abstract
Objective: Our objective was to model the potential cost-effectiveness of tirzepatide as an alternative to lifestyle modification (LSM) for the management of obesity and overweight.
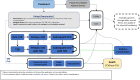
Methods: An individual-level discrete event simulation was implemented in Microsoft Excel linking short-term outcomes from the SURMOUNT-1 trial to key obesity-related complications to estimate costs and health benefits of tirzepatide (5-mg, 10-mg, or 15-mg doses) and LSM over a lifetime time horizon. Treatment-related changes in cardiometabolic factors were modeled using data from SURMOUNT-1; the relationship between patient status and risk of obesity complications was obtained from published literature. Modeled complications included cardiovascular events, onset of type 2 diabetes, cancer, osteoarthritis, and sleep apnea. The model simulated 1000 adult patients with overweight or obesity over their lifetimes, applying a 3% annual discount rate to cost and health outcomes. Only direct medical costs were considered.
Results: Tirzepatide 5, 10, and 15 mg provided 0.54, 0.55, and 0.61 additional quality-adjusted life years (QALYs) and additional costs of $79,288, $70,453, and $75,839 versus LSM, yielding incremental cost-effectiveness ratios of $146,331, $127,644, and $125,053 per QALY gained, respectively.
Conclusions: The model predicted that all doses of tirzepatide represent cost-effective alternatives to LSM for management of overweight and obesity at a willingness-to-pay threshold of $150,000 per QALY.
© 2025 Eli Lilly and Company. Obesity published by Wiley Periodicals LLC on behalf of The Obesity Society.
Conflict of interest statement
Kristen A. Deger, Sonja Sorensen, Ivan Houisse, and Mack S. Harris are employees of Evidera Inc., which received consulting fees from Eli Lilly and Company to support the research described in this paper. Meredith M. Hoog, Hong Kan, Jay Patrick Bae, Emily Ruth Hankosky, Donna Mojdami, Madhumita Murphy, and Lisa M. Neff are employees of Eli Lilly and Company and may hold shares and/or stock options in the company.
Figures

References
-
- Obesity Medicine Association (OMA) . Definition of obesity. Published August 29, 2017. Accessed October 25, 2022. https://obesitymedicine.org/definition-of-obesity/
-
- World Obesity Federation (WOF) . Obesity as a disease. Accessed October 25, 2022. https://www.worldobesity.org/what-we-do/our-policy-priorities/obesity-as....
-
- World Obesity Foundation . World Obesity Atlas 2022. https://www.worldobesityday.org/assets/downloads/World_Obesity_Atlas_202...
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

