Proteomic profiling reveals immunomodulatory role of IL-33 in ocular bacterial and fungal infections
- PMID: 40512033
- PMCID: PMC12234442
- DOI: 10.1128/iai.00183-25
Proteomic profiling reveals immunomodulatory role of IL-33 in ocular bacterial and fungal infections
Abstract
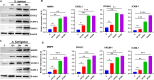
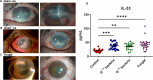
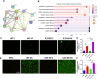
Bacterial and fungal pathogens are major causes of infectious endophthalmitis following eye surgery or trauma, often leading to vision impairment or blindness. The distinct clinical outcomes observed in bacterial and fungal endophthalmitis suggest differences in host immune responses. To investigate these differences, we utilized cytokine arrays and murine models of bacterial (Staphylococcus aureus) and fungal (Aspergillus fumigatus) endophthalmitis. Our analysis revealed that cytokine responses peaked in bacterial infections at 12-24 h, whereas fungal infections exhibited a delayed peak at 48 h. Several inflammatory mediators, including MMP9, MMP3, CD14, LIX, LCN2, retinol-binding protein 4, ICAM1, and VCAM1, were differentially elevated. Notably, interleukin-33 (IL-33) levels peaked early in bacterial infections but continued to rise throughout all time points in fungal endophthalmitis. Analysis of patient vitreous samples further confirmed higher levels of IL-33 in bacterial (n=40) and fungal (n=20) endophthalmitis cases. Functional studies in IL-33-deficient mice revealed an increased fungal burden and elevated TNF-α and IL-6 levels, but bacterial endophthalmitis severity remains largely unaffected. Additionally, bone marrow-derived macrophages from IL-33-/- mice exhibited increased cell death in response to fungal and bacterial infection. Our findings reveal divergent innate immune responses between bacterial and fungal endophthalmitis and emphasize the immunomodulatory function of IL-33 in ocular infections.
Keywords: IL-33; cell death; endophthalmitis; inflammation; proteomic profiler; retina.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Myeloid Cell-Specific Deletion of AMPKα1 Worsens Ocular Bacterial Infection by Skewing Macrophage Phenotypes.J Immunol. 2024 Dec 1;213(11):1656-1665. doi: 10.4049/jimmunol.2400282. J Immunol. 2024. PMID: 39413004
-
Global Transcriptomic Profiling of Innate and Adaptive Immunity During Aspergillus flavus Endophthalmitis in a Murine Model.Invest Ophthalmol Vis Sci. 2024 Apr 1;65(4):44. doi: 10.1167/iovs.65.4.44. Invest Ophthalmol Vis Sci. 2024. PMID: 38687493 Free PMC article.
-
Epidermal growth factor receptor signaling governs the host inflammatory response to invasive aspergillosis.mBio. 2024 Dec 11;15(12):e0267124. doi: 10.1128/mbio.02671-24. Epub 2024 Oct 30. mBio. 2024. PMID: 39475281 Free PMC article.
-
Adjunctive steroid therapy versus antibiotics alone for acute endophthalmitis after intraocular procedure.Cochrane Database Syst Rev. 2017 Feb 22;2(2):CD012131. doi: 10.1002/14651858.CD012131.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2022 Jun 6;6:CD012131. doi: 10.1002/14651858.CD012131.pub3. PMID: 28225198 Free PMC article. Updated.
-
Adjunctive steroid therapy versus antibiotics alone for acute endophthalmitis after intraocular procedure.Cochrane Database Syst Rev. 2022 Jun 6;6(6):CD012131. doi: 10.1002/14651858.CD012131.pub3. Cochrane Database Syst Rev. 2022. PMID: 35665485 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

