A chronic Pseudomonas aeruginosa mouse lung infection modeling the mucus obstruction, lung function, and inflammation of human cystic fibrosis
- PMID: 40512037
- PMCID: PMC12234440
- DOI: 10.1128/iai.00230-25
A chronic Pseudomonas aeruginosa mouse lung infection modeling the mucus obstruction, lung function, and inflammation of human cystic fibrosis
Abstract
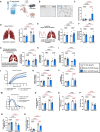
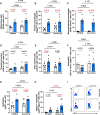
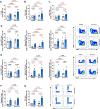
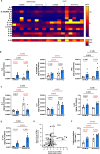
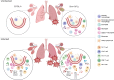
Mouse models of cystic fibrosis (CF) have been used to study chronic lung infections; however, these models have lacked the airway mucus that defines human CF pathophysiology and required the use of mucoid Pseudomonas aeruginosa. Alternative models have used either transgenic Scnn1b-Tg mice overexpressing a lung epithelial sodium channel to mimic the mucus-rich CF lung environment, synthetic CF sputum medium (SCFM2) to induce bacterial phenotypes consistent with human CF, or agar beads to promote chronic infections by non-mucoid P. aeruginosa. Here, we combined these alternative models and established a chronic P. aeruginosa lung infection model using SCFM2 agar beads and Scnn1b-Tg mice (SCFM2-Scnn1b-Tg) to recapitulate nutrient and mucus characteristics of the human CF lung environment and test the effects of chronic infections on bacterial burden, lung function, and the immune response. Using wild-type SCFM2-C57BL/6 mice as controls, SCFM2-Scnn1b-Tg mice failed to clear bacterial infections, and lung function measurements showed that infected SCFM2-Scnn1b-Tg mice had decreased inspiratory capacity and compliance, elevated airway resistance, and significantly reduced forced expiratory volumes. Flow cytometry and cytokine arrays showed that, like people with CF, SCFM2-Scnn1b-Tg mice developed inflammation characterized by neutrophil and eosinophil infiltration and Th2 lymphocytic cytokine responses. Chronically infected SCFM2-Scnn1b-Tg mice developed an exacerbated mix of innate and Th1, Th2, and Th17-mediated inflammation, causing higher lung cellular damage and elevated numbers of unusual Siglec F+ neutrophils. SCFM2-Scnn1b-Tg mice will be useful for investigating bacterial pathogenesis by non-mucoid P. aeruginosa, including treatments and the roles of Siglec F+ neutrophils in CF inflammation.
Keywords: Pseudomonas aeruginosa; animal models; cystic fibrosis; inflammation; lung infection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Update of
-
A chronic Pseudomonas aeruginosa mouse lung infection modeling the pathophysiology and inflammation of human cystic fibrosis.bioRxiv [Preprint]. 2024 Oct 7:2024.10.07.617039. doi: 10.1101/2024.10.07.617039. bioRxiv. 2024. Update in: Infect Immun. 2025 Jul 8;93(7):e0023025. doi: 10.1128/iai.00230-25. PMID: 39416002 Free PMC article. Updated. Preprint.
References
-
- Vaillancourt M, Galdino ACM, Limsuwannarot SP, Celedonio D, Dimitrova E, Broerman M, Bresee C, Doi Y, Lee JS, Parks WC, Jorth P. 2023. A compensatory RNase E variation increases iron piracy and virulence in multidrug-resistant Pseudomonas aeruginosa during macrophage infection. PLoS Pathog 19:e1010942. doi: 10.1371/journal.ppat.1010942 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

