Direction-dependent contributions of cardiac myofilament networks to myocardial passive stiffness reveal a major disparity for titin
- PMID: 40512239
- PMCID: PMC12325546
- DOI: 10.1007/s00395-025-01119-8
Direction-dependent contributions of cardiac myofilament networks to myocardial passive stiffness reveal a major disparity for titin
Abstract
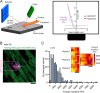
Progressive myocardial dysfunction in patients with heart failure often involves alterations in myocardial passive stiffness, yet the underlying mechanisms remain incompletely understood. While passive stiffness in the longitudinal direction has been extensively characterized via uniaxial tensile stretching of cardiac specimens, transverse stiffness has received far less attention despite its equal mechanical importance. In this study, we combined atomic force microscopy nanoindentation with stretching assays on myocardial preparations to quantify the relative contributions of the three myofilament networks - actin, myosin, and titin - to passive stiffness in both transverse and longitudinal orientations. We employed a transgenic mouse model in which titin's elastic springs contain a tobacco etch virus protease (TEVp) recognition site, enabling selective and acute titin cleavage upon TEVp treatment. Actin filaments were severed using a calcium-independent gelsolin fragment, and myosin filaments were dissociated by high-salt extraction. Along the longitudinal axis, titin accounted for over 50% of total passive stiffness in both cardiac fiber bundles and isolated cardiomyocytes across most physiological strain ranges, whereas actin contributed under 35% overall - and only 15-20% within the collagen-containing fiber bundles. In contrast, in the transverse axis, titin and actin each contributed approximately 20-26% of passive stiffness in cardiac slices under varying compression forces. The myosin-titin composite thick-filament network contributed ~ 55% longitudinally but only ~ 35% transversely. These results reveal pronounced, direction-dependent differences in myofilament contributions to myocardial passive stiffness, with titin exhibiting the greatest disparity. Our findings deepen our understanding of the myocardium's multidimensional mechanics and may inform therapeutic strategies to ameliorate pathological cardiac stiffening.
Keywords: Actin; Atomic force microscopy; Myocardium; Myosin; Passive stiffness; Titin.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: All authors declare that they have no conflict of interest related to this manuscript. Ethics: We obtained permission for all animal procedures from the local animal welfare authority (Landesamt für Natur, Umwelt und Verbraucherschutz Nordrhein-Westfalen, LANUV NRW, 81–02.04.2019.A472). The manuscript does not contain clinical studies or patient data.
Figures




References
-
- Armstrong PW, Lam CSP, Anstrom KJ, Ezekowitz J, Hernandez AF, O’Connor CM, Pieske B, Ponikowski P, Shah SJ, Solomon SD, Voors AA, She L, Vlajnic V, Carvalho F, Bamber L, Blaustein RO, Roessig L, Butler J (2020) Effect of vericiguat vs placebo on quality of life in patients with heart failure and preserved ejection fraction: the VITALITY-HFpEF randomized clinical trial. JAMA 324:1512–1521. 10.1001/jama.2020.15922 - DOI - PMC - PubMed
-
- Bishu K, Hamdani N, Mohammed SF, Kruger M, Ohtani T, Ogut O, Brozovich FV, Burnett JC Jr, Linke WA, Redfield MM (2011) Sildenafil and B-type natriuretic peptide acutely phosphorylate titin and improve diastolic distensibility in vivo. Circulation 124:2882–2891. 10.1161/CIRCULATIONAHA.111.048520 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

