Nose to brain delivery of nanostructured lipid carriers loaded with rivastigmine and nilotinib for treating Alzheimer's disease: preparation, cell line study, and in vivo evaluation
- PMID: 40512424
- PMCID: PMC12165938
- DOI: 10.1186/s11671-025-04276-w
Nose to brain delivery of nanostructured lipid carriers loaded with rivastigmine and nilotinib for treating Alzheimer's disease: preparation, cell line study, and in vivo evaluation
Abstract
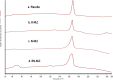
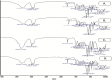
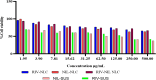
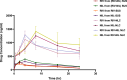
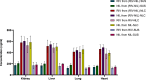
Alzheimer's disease (AD) is a complex neurodegenerative that affects over 55 million people worldwide, a number expected to double by 2050 due to aging populations. This growing prevalence imposes immense societal and economic burdens on healthcare systems and caregivers. AD is challenging to treat with monotherapy, making combination therapy a more effective approach. This study focuses on delivering Rivastigmine tartrate (RIV), and Nilotinib hydrochloride monohydrate (NIL), to the brain to achieve synergistic effects against AD. The optimal ratio of the drug combination was determined using the combination index that was performed using the Neuro2a cells line. It was found to be 1:1, emphasizing the synergistic effect against the cell lines. So, nanostructured lipid carriers (NLCs) were loaded with RIV and NIL, both individually and in combination, developed and optimized in this study. The developed formulations were thoroughly characterized for globule size, polydispersity index (PDI), and entrapment efficiency (EE) for each drug and the combination. The globule size was > 200 nm, PDI > 0.3; EE < 85% in all the developed formulations. On performing an in vitro cell availability study it was found that developed NLCs showed a 1.3 to 1.4-fold increase in the viability of the cells. On conducting an in vivo study, the concentration in the brain following administration of different formulations was in the order of RIV-NIL-NLC > NIL-NLC > RIV-NLC > RIV-NIL SUS > NIL-SUS > RIV-SUS. There was a 3.5 to 5-fold increase in the concentration of RIV and NIL in the brain when administered as RIV-NIL-NLC. So, it can be concluded that the NLCs with combined drugs showed promising results, enhancing drug permeability through the intranasal route, therefore could be used for treating AD.
Keywords: Alzheimer’s disease; Combination drugs; Nanostructured lipid carrier; Nilotinib hydrochloride monohydrate; Pharmacokinetic study; Rivastigmine tartrate.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Institutional review board statement: The animal study protocol was permitted by the Institutional Animal Ethical Committee (IAEC) (Jamia Hamdard, New Delhi, India).
Figures








Similar articles
-
Double Optimization of Rivastigmine-Loaded Nanostructured Lipid Carriers (NLC) for Nose-to-Brain Delivery Using the Quality by Design (QbD) Approach: Formulation Variables and Instrumental Parameters.Pharmaceutics. 2020 Jun 28;12(7):599. doi: 10.3390/pharmaceutics12070599. Pharmaceutics. 2020. PMID: 32605177 Free PMC article.
-
Rivastigmine-DHA ion-pair complex improved loading in hybrid nanoparticles for better amyloid inhibition and nose-to-brain targeting in Alzheimer's.Eur J Pharm Biopharm. 2023 Sep;190:131-149. doi: 10.1016/j.ejpb.2023.06.007. Epub 2023 Jun 16. Eur J Pharm Biopharm. 2023. PMID: 37330117
-
Nose to Brain Delivery of Astaxanthin-Loaded Nanostructured Lipid Carriers in Rat Model of Alzheimer's Disease: Preparation, in vitro and in vivo Evaluation.Int J Nanomedicine. 2023 Mar 30;18:1631-1658. doi: 10.2147/IJN.S402447. eCollection 2023. Int J Nanomedicine. 2023. PMID: 37020692 Free PMC article.
-
Navigating the Nose-to-Brain Route: A Systematic Review on Lipid-Based Nanocarriers for Central Nervous System Disorders.Pharmaceutics. 2024 Feb 27;16(3):329. doi: 10.3390/pharmaceutics16030329. Pharmaceutics. 2024. PMID: 38543223 Free PMC article. Review.
-
Improving Drug Delivery for Alzheimer's Disease Through Nose-to-Brain Delivery Using Nanoemulsions, Nanostructured Lipid Carriers (NLC) and in situ Hydrogels.Int J Nanomedicine. 2021 Jun 29;16:4373-4390. doi: 10.2147/IJN.S305851. eCollection 2021. Int J Nanomedicine. 2021. PMID: 34234432 Free PMC article. Review.
References
-
- Sheppard O, Coleman M. Alzheimer’s disease: etiology, neuropathology and pathogenesis. Alzheimer’s Disease Drug Discov. 2020. 10.36255/EXONPUBLICATIONS.ALZHEIMERSDISEASE.2020.CH1.
-
- Nichols E, Steinmetz JD, Vollset SE, et al. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the global burden of disease study 2019. Lancet Public Health. 2022;7:e105–25. 10.1016/S2468-2667(21)00249-8/ATTACHMENT/60E03FD1-38B2-4B40-A91D-9AFDDA22B45E/MMC1.PDF. - PMC - PubMed
-
- FDA approves treatment for adults with Alzheimer’s disease | FDA. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-treatment.... Accessed 8 Jan 2025
-
- Moura RP, Pacheco C, Pêgo AP, et al. Lipid nanocapsules to enhance drug bioavailability to the central nervous system. J Control Release. 2020;322:390–400. 10.1016/J.JCONREL.2020.03.042. - PubMed
LinkOut - more resources
Full Text Sources
