AI-Assisted vs Unassisted Identification of Prostate Cancer in Magnetic Resonance Images
- PMID: 40512493
- PMCID: PMC12166490
- DOI: 10.1001/jamanetworkopen.2025.15672
AI-Assisted vs Unassisted Identification of Prostate Cancer in Magnetic Resonance Images
Abstract
Importance: Artificial intelligence (AI) assistance in magnetic resonance imaging (MRI) assessment for prostate cancer shows promise for improving diagnostic accuracy but lacks large-scale observational evidence.
Objective: To evaluate whether use of AI-assisted assessment for diagnosing clinically significant prostate cancer (csPCa) on MRI is superior to unassisted readings.
Design, setting, and participants: This diagnostic study was conducted between March and July 2024 to compare unassisted and AI-assisted diagnostic performance using the AI system developed within the international Prostate Imaging-Cancer AI (PI-CAI) Consortium. The study involved 61 readers (34 experts and 27 nonexperts) from 53 centers across 17 countries. Readers assessed prostate magnetic resonance images both with and without AI assistance, providing Prostate Imaging Reporting and Data System (PI-RADS) annotations from 3 to 5 (higher PI-RADS indicated a higher likelihood of csPCa) and patient-level suspicion scores ranging from 0 to 100 (higher scores indicated a greater likelihood of harboring csPCa). Biparametric prostate MRI examinations were included for 780 men from the PI-CAI study who were included in the newly-conducted observer study. All men within the PI-CAI study had suspicion of harboring prostate cancer, sufficient diagnostic image quality, and no prior clinically significant cancer findings. Disease presence was defined by histopathology, and absence was determined by 3 or more years of follow-up. The AI system was recalibrated using 420 Dutch examinations to generate lesion-detection maps, with AI scores ranging from 1 to 10, in which 10 indicates the highest likelihood of csPCa. The remaining 360 examinations, originating from 3 Dutch centers and 1 Norwegian center, were included in the observer study.
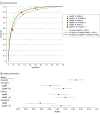
Main outcomes and measures: The primary outcome was diagnosis of csPCa, evaluated using the area under the receiver operating characteristic curve and sensitivity and specificity at a PI-RADS threshold of 3 or more. The secondary outcomes included analysis at alternate operating points and reader expertise.
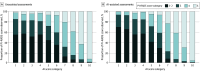
Results: Among the 360 examinations of 360 men (median age, 65 years [IQR, 62-70 years]) who were included for testing, 122 (34%) harbored csPCa. AI assistance was associated with significantly improved performance, achieving a 3.3% increase in the area under the receiver operating characteristic curve (95% CI, 1.8%-4.9%; P < .001), from 0.882 (95% CI, 0.854-0.910) in unassisted assessments to 0.916 (95% CI, 0.893-0.938) with AI assistance. Sensitivity improved by 2.5% (95% CI, 1.1%-3.9%; P < .001), from 94.3% (95% CI, 91.9%-96.7%) to 96.8% (95% CI, 95.2%-98.5%), and specificity increased by 3.4% (95% CI, 0.8%-6.0%; P = .01), from 46.7% (95% CI, 39.4%-54.0%) to 50.1% (95% CI, 42.5%-57.7%), at a PI-RADS score of 3 or more. Secondary analyses demonstrated similar performance improvements across alternate operating points and a greater benefit of AI assistance for nonexpert readers.
Conclusions and relevance: The findings of this diagnostic study of patients suspected of harboring prostate cancer suggest that AI assistance was associated with improved radiologic diagnosis of clinically significant disease. Further research is required to investigate the generalization of outcomes and effects on workflow improvement within prospective settings.
Conflict of interest statement
Figures

Comment in
-
Artificial Intelligence-Assisted Interpretation of Prostate MRI Improves Cancer Detection.AJR Am J Roentgenol. 2025 Sep 17. doi: 10.2214/AJR.25.33877. Online ahead of print. AJR Am J Roentgenol. 2025. PMID: 40960249 No abstract available.
References
-
- van der Leest M, Cornel E, Israël B, et al. Head-to-head comparison of transrectal ultrasound-guided prostate biopsy versus multiparametric prostate resonance imaging with subsequent magnetic resonance-guided biopsy in biopsy-naïve men with elevated prostate-specific antigen: a large prospective multicenter clinical study. Eur Urol. 2019;75(4):570-578. doi: 10.1016/j.eururo.2018.11.023 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

