Isolation of Verrucosins A-E from a Marine Verrucosispora sp. Reveals a Unifying Biosynthetic Hypothesis for Linear and Macrocyclic Polyketides
- PMID: 40512498
- PMCID: PMC12305660
- DOI: 10.1021/acs.jnatprod.5c00373
Isolation of Verrucosins A-E from a Marine Verrucosispora sp. Reveals a Unifying Biosynthetic Hypothesis for Linear and Macrocyclic Polyketides
Abstract
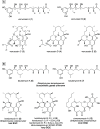
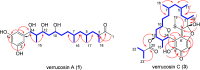
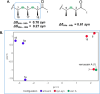
As part of our long-standing program evaluating the biosynthetic complexity and biomedical potential of natural products from marine microbes, our attention was drawn to culture extracts from a Verrucosispora sp. (strain TAA-831), which produced multiple compounds with unique UV absorbance signatures and HRMS data. Large-scale fermentation and targeted isolation afforded verrucosins A-E (1-5), a mixture of linear and macrocyclic polyketides whose structures were determined through a synergistic combination of experimental, computational, and genomic approaches. The conserved sequence of methyl malonate and malonate motifs across the verrucosins implied a shared biosynthetic origin despite structural divergence as linear and cyclic congeners. Targeted genome mining revealed a lone type I/type III hybrid polyketide synthase biosynthetic gene cluster, vrs, that is likely responsible for verrucosin production. This revelation demonstrates for the first time that linear 3,5-dihydroxybenzenic (1 and 2) and cyclic ansamycin (3-5) polyketides can be naturally produced by a single biosynthetic gene cluster. The identification of the vrs cluster and bioinformatic prediction of the stereoselectivity of the embedded reductive domains within the modular type I polyketide synthase reinforced the NMR and computational stereochemical assignments for the co-isolates, particularly the stereochemically complex linear verrucosins (1 and 2).
Figures



Similar articles
-
Discovery of Tricyclic Aromatic Polyketides Reveals Hidden Chain-Length Flexibility in Type II Polyketide Synthases.Int J Mol Sci. 2025 Aug 13;26(16):7801. doi: 10.3390/ijms26167801. Int J Mol Sci. 2025. PMID: 40869122 Free PMC article.
-
Genome-based discovery of polyketides generated by trans-acyltransferase polyketide synthases.Methods Enzymol. 2025;717:317-347. doi: 10.1016/bs.mie.2025.03.002. Epub 2025 Apr 22. Methods Enzymol. 2025. PMID: 40651830 Review.
-
Tailoring of Prematurely Released Polyketide Intermediates in Piericidin Biosynthesis.J Nat Prod. 2025 Aug 22;88(8):1928-1935. doi: 10.1021/acs.jnatprod.5c00609. Epub 2025 Jul 27. J Nat Prod. 2025. PMID: 40717309 Free PMC article.
-
Structural insights and rational engineering strategies for modular polyketide synthases: A review.Int J Biol Macromol. 2025 Aug;319(Pt 3):145299. doi: 10.1016/j.ijbiomac.2025.145299. Epub 2025 Jun 16. Int J Biol Macromol. 2025. PMID: 40533022 Review.
-
Bioactive Polyketides from Amphidinium spp.: An In-Depth Review of Biosynthesis, Applications, and Current Research Trends.Mar Drugs. 2025 Jun 16;23(6):255. doi: 10.3390/md23060255. Mar Drugs. 2025. PMID: 40559664 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

