De-coupling immune parameters and toxicity associated with IL-12 agonism
- PMID: 40512622
- PMCID: PMC12264503
- DOI: 10.1016/j.celrep.2025.115840
De-coupling immune parameters and toxicity associated with IL-12 agonism
Abstract
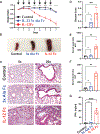
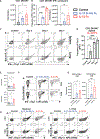
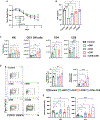
Interleukin-12 (IL-12) stimulates natural killer (NK) and T cell production of interferon gamma (IFN-γ), but adverse events from NK cell activation have limited its clinical use. This study shows the impact of half-life-extended, full (IL-12Fc) and partial (IL-12 3x AlaFc) IL-12 agonists on the immune system. In naive mice, serial treatment with IL-12Fc induces systemic IFN-γ, multi-organ pathology, and alterations in myelopoiesis. IL-12 Fc stimulates NK cell production of IFN-γ but also activates CD4+, CD8+ T, and NKT cells. IL-12 Fc's ability to enhance the production of IFN-γ facilitates myelopoiesis, but IFN-γ is not required for the development of systemic toxicity. In contrast, IL-12 3x Ala Fc avoids overt disease, activates CD4+ and CD8+ T cells, and induces myelopoiesis. These differential activities were harnessed to enhance resistance to infection, indicating that a threshold of IL-12 signaling is tolerated under steady-state conditions and that fine-tuning IL-12 agonism can bolster resistance without triggering pathology.
Keywords: CP: Immunology; hematopoeisis; infection; innate and adaptive immunity; interleukin-12; toxicity.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests B.J. and P.L. are current employees and shareholders of Synthekine, Inc., and C.A. Hunter is on the scientific advisory board of Synthekine, Inc.
Figures

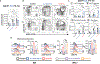


Similar articles
-
IL-36/IL-36R signaling promotes CD4+ T cell-dependent colitis via pro-inflammatory cytokine production.Front Immunol. 2025 Jun 26;16:1604332. doi: 10.3389/fimmu.2025.1604332. eCollection 2025. Front Immunol. 2025. PMID: 40642091 Free PMC article.
-
Biological sex and age influence GS-9620 activity ex vivo.JCI Insight. 2025 May 6;10(12):e182242. doi: 10.1172/jci.insight.182242. eCollection 2025 Jun 23. JCI Insight. 2025. PMID: 40327405 Free PMC article.
-
CD4 T-cell expression of IFN-γ and IL-17 in pediatric malarial anemia.PLoS One. 2017 Apr 20;12(4):e0175864. doi: 10.1371/journal.pone.0175864. eCollection 2017. PLoS One. 2017. PMID: 28426727 Free PMC article.
-
Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation.Health Technol Assess. 2006 Aug;10(28):iii-iv, xi-xiv, 1-183. doi: 10.3310/hta10280. Health Technol Assess. 2006. PMID: 16904047
-
Identification of Parameters Representative of Immune Dysfunction in Patients with Severe and Fatal COVID-19 Infection: a Systematic Review and Meta-analysis.Clin Rev Allergy Immunol. 2023 Feb;64(1):33-65. doi: 10.1007/s12016-021-08908-8. Epub 2022 Jan 18. Clin Rev Allergy Immunol. 2023. PMID: 35040086 Free PMC article.
References
-
- Gazzinelli RT, Hieny S, Wynn TA, Wolf S, and Sher A (1993). Interleukin 12 is required for the T-lymphocyte-independent induction of interferon gamma by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc. Natl. Acad. Sci. USA 90, 6115–6119. 10.1073/pnas.90.13.6115. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

