Fast photostable expansion microscopy using QDots and deconvolution
- PMID: 40512684
- PMCID: PMC12165338
- DOI: 10.1371/journal.pone.0325155
Fast photostable expansion microscopy using QDots and deconvolution
Abstract
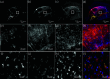
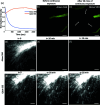
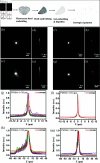
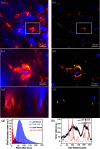
Expansion microscopy (ExM) enables sub-diffraction imaging by physically expanding labeled tissue samples. This increases the tissue volume relative to the instrument point spread function (PSF), thereby improving the effective resolution by reported factors of 4 - 20X. However, this volume increase dilutes the fluorescence signal, reducing both signal-to-noise ratio (SNR) and acquisition speed. This paper proposes and validates a method for mitigating these challenges. We overcame the limitations of ExM by developing a fast photo-stable protocol to enable scalable widefield three-dimensional imaging with ExM. We combined widefield imaging with quantum dots (QDots). Widefield imaging provides a significantly faster acquisition of a single field-of-view (FOV). However, the uncontrolled incoherent illumination induces photobleaching. We mitigated this challenge using QDots, which exhibit a long fluorescence lifetime and improved photostability. First, we developed a protocol for QDot labeling. Next, we utilized widefield imaging to obtain 3D image stacks and applied deconvolution, which is feasible due to reduced scattering in ExM samples. We show that increased transparency, which is a side-effect of ExM, enables widefield deconvolution, dramatically reducing the acquisition time for three-dimensional images compared to laser scanning microscopy. The proposed QDot labeling protocol is compatible with ExM and provides enhanced photostability compared to traditional fluorescent dyes. Widefield imaging significantly improves SNR and acquisition speed compared to conventional confocal microscopy. Combining widefield imaging with QDot labeling and deconvolution has the potential to be applied to ExM for faster imaging of large three-dimensional samples with improved SNR.
Copyright: © 2025 Gunawardhana et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
Jason Eriksen and David Mayerich have an ownership stake in SwiftFront, LLC. Wilna Moree is an employee of SwiftFront, LLC and was provided salary support. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures





Update of
-
Fast Photostable Expansion Microscopy Using QDots and Deconvolution.bioRxiv [Preprint]. 2024 Nov 25:2024.11.25.625167. doi: 10.1101/2024.11.25.625167. bioRxiv. 2024. Update in: PLoS One. 2025 Jun 13;20(6):e0325155. doi: 10.1371/journal.pone.0325155. PMID: 39651290 Free PMC article. Updated. Preprint.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

