Top-down attention and Alzheimer's pathology affect cortical selectivity during learning, influencing episodic memory in older adults
- PMID: 40512843
- PMCID: PMC12164959
- DOI: 10.1126/sciadv.ads4206
Top-down attention and Alzheimer's pathology affect cortical selectivity during learning, influencing episodic memory in older adults
Abstract
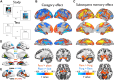
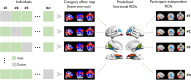
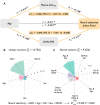
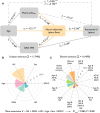
Effective memory formation declines in human aging. Diminished neural selectivity-reduced differential responses to preferred versus nonpreferred stimuli-may contribute to memory decline, but its drivers remain unclear. We investigated the effects of top-down attention and preclinical Alzheimer's disease (AD) pathology on neural selectivity in 166 cognitively unimpaired older participants using functional magnetic resonance imaging during a word-face/word-place associative memory task. During learning, neural selectivity in place- and, to a lesser extent, face-selective regions was greater for subsequently remembered than forgotten events; positively scaled with variability in dorsal attention network activity, within and across individuals; and negatively related to AD pathology, evidenced by elevated plasma phosphorylated Tau181 (pTau181). Path analysis revealed that neural selectivity mediated the effects of age, attention, and pTau181 on memory. These data reveal multiple pathways that contribute to memory differences among older adults-AD-independent reductions in top-down attention and AD-related pathology alter the precision of cortical representations of events during experience, with consequences for remembering.
Figures


Update of
-
Top-down attention and Alzheimer's pathology impact cortical selectivity during learning, influencing episodic memory in older adults.bioRxiv [Preprint]. 2024 Dec 9:2024.12.04.626911. doi: 10.1101/2024.12.04.626911. bioRxiv. 2024. Update in: Sci Adv. 2025 Jun 13;11(24):eads4206. doi: 10.1126/sciadv.ads4206. PMID: 39713293 Free PMC article. Updated. Preprint.
Similar articles
-
Top-down attention and Alzheimer's pathology impact cortical selectivity during learning, influencing episodic memory in older adults.bioRxiv [Preprint]. 2024 Dec 9:2024.12.04.626911. doi: 10.1101/2024.12.04.626911. bioRxiv. 2024. Update in: Sci Adv. 2025 Jun 13;11(24):eads4206. doi: 10.1126/sciadv.ads4206. PMID: 39713293 Free PMC article. Updated. Preprint.
-
Differential effects of aging, Alzheimer's pathology, and APOE4 on longitudinal functional connectivity and episodic memory in older adults.Alzheimers Res Ther. 2025 Apr 25;17(1):91. doi: 10.1186/s13195-025-01742-6. Alzheimers Res Ther. 2025. PMID: 40281595 Free PMC article.
-
Neuroimaging Predictors of Cognitive Resilience against Alzheimer's Disease Pathology.Ann Neurol. 2025 Jun;97(6):1038-1050. doi: 10.1002/ana.27186. Epub 2025 Jan 31. Ann Neurol. 2025. PMID: 39891430 Free PMC article.
-
Medial temporal lobe connectivity and its associations with cognition in early Alzheimer's disease.Brain. 2020 Apr 1;143(4):1233-1248. doi: 10.1093/brain/awaa068. Brain. 2020. PMID: 32252068 Free PMC article.
-
Association of CSF Biomarkers With Hippocampal-Dependent Memory in Preclinical Alzheimer Disease.Neurology. 2021 Mar 9;96(10):e1470-e1481. doi: 10.1212/WNL.0000000000011477. Epub 2021 Jan 6. Neurology. 2021. PMID: 33408146 Free PMC article.
Cited by
-
Synergistic effects of APOE ε4 and Alzheimer's pathology on the neural correlates of episodic remembering in cognitively unimpaired older adults.bioRxiv [Preprint]. 2025 Jun 25:2025.06.20.660774. doi: 10.1101/2025.06.20.660774. bioRxiv. 2025. PMID: 40667041 Free PMC article. Preprint.
References
-
- M. J. Kahana, A. D. Wagner, Oxford Handbook of Human Memory (Oxford Univ. Press, 2024); https://memory.psych.upenn.edu/Oxford_Handbook_of_Human_Memory.
-
- Cabeza R., Albert M., Belleville S., Craik F. I. M., Duarte A., Grady C. L., Lindenberger U., Nyberg L., Park D. C., Reuter-Lorenz P. A., Rugg M. D., Steffener J., Rajah M. N., Maintenance, reserve and compensation: The cognitive neuroscience of healthy ageing. Nat. Rev. Neurosci. 19, 701–710 (2018). - PMC - PubMed
-
- Mormino E. C., Kluth J. T., Madison C. M., Rabinovici G. D., Baker S. L., Miller B. L., Koeppe R. A., Mathis C. A., Weiner M. W., Jagust W. J., Alzheimer’s Disease Neuroimaging Initiative , Episodic memory loss is related to hippocampal-mediated β-amyloid deposition in elderly subjects. Brain 132, 1310–1323 (2009). - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

