Regulation of PIEZO1 channel force sensitivity by interblade handshaking
- PMID: 40512861
- PMCID: PMC12164982
- DOI: 10.1126/sciadv.adt7046
Regulation of PIEZO1 channel force sensitivity by interblade handshaking
Abstract
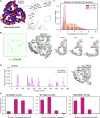
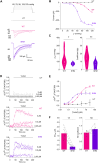
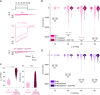
PIEZOs form trimeric calcium-permeable nonselective cationic channels that serve mechanical sensing needs across eukaryotic biology. Forces act on the channels by causing their curved blades to flatten and decompact, leading to an activated state, but it is unclear how this is regulated to enable the channels to adapt to different contexts. To identify potential mechanisms, we performed coarse-grained and all-atom molecular dynamics simulations on human PIEZO1. We observed an interblade handshake interaction mediated by basic amino acid residues in two flexible helices coordinated with regulated anionic lipid phosphatidylinositol 4,5-bisphosphate. The interaction determined the resting configuration of the channel, blade curvature, compactness, and ion pore structure. In experiments, disruption of the handshake by neutralization of helix amino acids or phosphatidylinositol 4,5-bisphosphate depletion increased the channel's sensitivity to membrane tension. Structural and amino acid sequence analysis for multiple PIEZOs predicted helix amino acid arrangements for varied handshaking intensity. We suggest a dynamic interaction in PIEZO channels that regulates force sensitivity.
Figures




References
-
- Li J., Hou B., Tumova S., Muraki K., Bruns A., Ludlow M. J., Sedo A., Hyman A. J., McKeown L., Young R. S., Yuldasheva N. Y., Majeed Y., Wilson L. A., Rode B., Bailey M. A., Kim H. R., Fu Z., Carter D. A. L., Bilton J., Imrie H., Ajuh P., Dear T. N., Cubbon R. M., Kearney M. T., Prasad K. R., Evans P. C., Ainscough J. F. X., Beech D. J., Piezo1 integration of vascular architecture with physiological force. Nature 515, 279–282 (2014). - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

