Mutation of an active site-adjacent residue in VIM indirectly dictates interactions with and blunts inhibition by D-captopril
- PMID: 40513263
- PMCID: PMC12218919
- DOI: 10.1016/j.jinorgbio.2025.112975
Mutation of an active site-adjacent residue in VIM indirectly dictates interactions with and blunts inhibition by D-captopril
Abstract
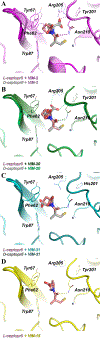
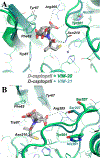
Activity assays and X-ray crystallographic studies were undertaken to elucidate the inhibitory mechanism of captopril stereoisomers on Verona integron-encoded metallo-β-lactamases, specifically VIM-20, VIM-31, and VIM-15. All three VIM-2-like variants (VIM-20, VIM-31, and VIM-15) and VIM-2 expressed in Escherichia coli exhibited catalytic activity with comparable steady-state kinetic parameters. Among the tested thiol drugs (L- and D-captopril, D,L-thiorphan, and 2,3-dimercaprol), IC50 analyses indicated that D-captopril and 2,3-dimercaprol were more potent inhibitors against the VIM enzymes examined in this study. Notably, the IC50 value of D-captopril against VIM-31 was an exception, closely resembling that of L-captopril. To elucidate this exceptional inhibitory potency of D-captopril and its binding mode in the active site of VIM-31, high-resolution crystal structures of VIM-20, VIM-31, and VIM-15 in complex with both L- and D-captopril are reported. These findings will help evaluate whether the identified potent inhibitor D-captopril could be further developed as a pan inhibitor targeting the VIM-family enzymes.
Keywords: Antibiotic resistance; Enzyme inhibition; Metallo-β-lactamase; Protein crystallography.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest Richard Page reports financial support was provided by National Institute of General Medical Sciences. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Pandey N, Cascella M (2023) Beta-Lactam Antibiotics. In: StatPearls. StatPearls Publishing, Treasure Island (FL) - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

