Diet-incorporated saracatinib, a Src tyrosine kinase inhibitor, counteracts diisopropylfluorophosphate (DFP)-induced chronic neurotoxicity in the rat model
- PMID: 40513390
- PMCID: PMC12289090
- DOI: 10.1016/j.biopha.2025.118234
Diet-incorporated saracatinib, a Src tyrosine kinase inhibitor, counteracts diisopropylfluorophosphate (DFP)-induced chronic neurotoxicity in the rat model
Abstract
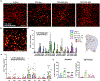
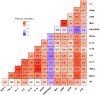
Acute exposure to diisopropylfluorophosphate (DFP), an organophosphate nerve agent, induces status epilepticus (SE) and epileptogenesis despite treatment with countermeasures immediately after exposure. Epileptogenesis is characterized by increased Src family tyrosine kinases (SFK), nitrooxidative stress, reactive gliosis, and neurodegeneration in the early stage, followed by spontaneous seizures at a later stage. We hypothesized that treating with saracatinib (SAR), an SFK inhibitor, would mitigate the early markers of epileptogenesis. Therefore, in this study, we investigated the effects of SAR in the diet (10-20 mg/kg/day, high-dose or 5-10 mg/kg/day, low-dose) fed for four weeks post-DFP. SAR-in-diet significantly mitigated DFP-induced nitrooxidative stress markers (nitrite, GSH/GSSG) and pro-inflammatory cytokines/chemokine (TNF-α, IL-17, IL-6, IL-18, IL-1α, MCP-1) in serum and cerebrospinal fluid. DFP-exposed rats exhibited increased reactive microglia (IBA1 +CD68) and reactive astrocytes (GFAP+C3) in extrahippocampal and thalamic regions. Both SAR dosing regimens significantly reduced reactive astrogliosis across several brain regions in both sexes, while the low-dose SAR-in-diet significantly reduced both microgliosis and reactive microgliosis in the laterodorsal thalamic (LDT) nucleus in males. Sex-specific effects of SAR at high-dose were observed in astrogliosis in females in the dentate gyrus, subiculum, amygdala, and LDT. Both SAR dosing regimens significantly reduced neurodegeneration (FJB-positive neurons) in males in the centromedian thalamic nucleus. Pearson correlation analyses revealed strong associations between key epileptogenic markers and SAR-in-diet treatment. These findings underscore the complex relationship between the early markers of epileptogenesis and the disease-modifying potential of SAR-in-diet. Additionally, the SAR-in-diet treatment approach is translational and reduces handling stress in animals in long-term studies.
Keywords: Diisopropylfluorophosphate (DFP) model; Key proinflammatory cytokines; Neuroinflammation; Nitro-oxidative stress; Organophosphate nerve agents; Saracatinib (AZD0530).
Copyright © 2025 The Authors. Published by Elsevier Masson SAS.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest None of the authors of the manuscript have any conflicts of interest.
Figures







Similar articles
-
Mitigating organophosphate nerve agent, soman (GD), induced long-term neurotoxicity: Saracatinib, a Src Tyrosine Kinase inhibitor, as a potential countermeasure.J Neuroinflammation. 2025 Aug 5;22(1):199. doi: 10.1186/s12974-025-03520-5. J Neuroinflammation. 2025. PMID: 40764938 Free PMC article.
-
Mitigating Organophosphate Nerve Agent, Soman (GD)-Induced Long-Term Neurotoxicity: Saracatinib, a Src Tyrosine Kinase Inhibitor, as a Potential Countermeasure.Res Sq [Preprint]. 2025 Jun 24:rs.3.rs-6674766. doi: 10.21203/rs.3.rs-6674766/v1. Res Sq. 2025. Update in: J Neuroinflammation. 2025 Aug 5;22(1):199. doi: 10.1186/s12974-025-03520-5. PMID: 40678225 Free PMC article. Updated. Preprint.
-
Spatiotemporal perturbations of the plasminogen activation system in a rat model of acute organophosphate intoxication.Acta Neuropathol Commun. 2025 Mar 18;13(1):62. doi: 10.1186/s40478-025-01979-0. Acta Neuropathol Commun. 2025. PMID: 40102979 Free PMC article.
-
Persistent behavior deficits, neuroinflammation, and oxidative stress in a rat model of acute organophosphate intoxication.Neurobiol Dis. 2020 Jan;133:104431. doi: 10.1016/j.nbd.2019.03.019. Epub 2019 Mar 21. Neurobiol Dis. 2020. PMID: 30905768 Free PMC article. Review.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
Cited by
-
Assessment of pharmacokinetics, safety, and neuroprotective efficacy of an adjunct intramuscular verapamil therapy in a rat model of organophosphate DFP-induced status epilepticus.bioRxiv [Preprint]. 2025 Jun 27:2025.06.25.661332. doi: 10.1101/2025.06.25.661332. bioRxiv. 2025. PMID: 40666862 Free PMC article. Preprint.
-
Mitigating organophosphate nerve agent, soman (GD), induced long-term neurotoxicity: Saracatinib, a Src Tyrosine Kinase inhibitor, as a potential countermeasure.J Neuroinflammation. 2025 Aug 5;22(1):199. doi: 10.1186/s12974-025-03520-5. J Neuroinflammation. 2025. PMID: 40764938 Free PMC article.
References
-
- Massey N, Vasanthi SS, Samidurai M, Gage M, Rao N, Meyer C, Thippeswamy T, 1400 W, a selective inducible nitric oxide synthase inhibitor, mitigates early neuroinflammation and nitrooxidative stress in diisopropylfluorophosphate-induced short-term neurotoxicity rat model, Front Mol. Neurosci 16 (2023) 1125934. - PMC - PubMed
-
- Adeyinka A, Muco E, Regina AC, Pierre L, Organophosphates, StatPearls, 2024. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

