Spatial heterogeneity of PD-L1 expression influence its assessment in esophageal squamous cell carcinoma
- PMID: 40513524
- PMCID: PMC12205590
- DOI: 10.1016/j.tranon.2025.102442
Spatial heterogeneity of PD-L1 expression influence its assessment in esophageal squamous cell carcinoma
Abstract
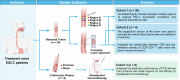
Immune checkpoint inhibitors are a promising treatment for esophageal squamous cell carcinoma (ESCC). However, the predictive value of programmed death-ligand 1 (PD-L1) expression, the most common biomarker for immunotherapy, remains controversial, particularly in the neoadjuvant setting. We hypothesized that the spatial heterogeneous of PD-L1 expression within tumors might render limited biopsy samples unrepresentative of the bulk tumor. In this study, we assessed the spatial heterogeneity in PD-L1 expression within ESCC by sampling four distinct regions using endoscopic biopsy forceps and the largest longitudinal sections on complete resected tumor from treatment-naïve patients. Our findings demonstrated the insufficiency of using limited biopsy tumor tissue to accurately determine the combined positive score (CPS) within the tumor. Notably, spatial heterogeneity was reduced when tumor's CPS was sufficiently high. Multi-region sampling assessment revealed that the maximum CPS derived from three regions provided a more accurate approximation of the bulk tumor's PD-L1 status. Additionally, the densities of CD8+/CD4+T cells were positively correlated with CPS. These findings emphasize the clinical need for standardized and modified biopsy assessment strategies to improve the accuracy of PD-L1 evaluation, thereby guiding therapeutic decision-making in ESCC.
Keywords: Endoscopic biopsy; Esophageal squamous cell carcinoma; Immunotherapy; PD-L1; Spatial heterogeneity.
Copyright © 2025. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest The authors declare no potential conflicts of interest.
Figures





Similar articles
-
Prognostic value of PD-L1 expression on immune cells or tumor cells for locally advanced esophageal squamous cell carcinoma in patients treated with neoadjuvant chemoradiotherapy.J Cancer Res Clin Oncol. 2022 Jul;148(7):1803-1811. doi: 10.1007/s00432-021-03772-7. Epub 2021 Aug 25. J Cancer Res Clin Oncol. 2022. PMID: 34432128 Free PMC article.
-
Oncolytic reovirus enhances the effect of CEA immunotherapy when combined with PD1-PDL1 inhibitor in a colorectal cancer model.Immunotherapy. 2025 Apr;17(6):425-435. doi: 10.1080/1750743X.2025.2501926. Epub 2025 May 12. Immunotherapy. 2025. PMID: 40353308
-
Rationale and feasibility of a rapid integral biomarker program that informs immune-oncology clinical trials: the ADVISE trial.J Immunother Cancer. 2025 May 19;13(5):e011170. doi: 10.1136/jitc-2024-011170. J Immunother Cancer. 2025. PMID: 40389374 Free PMC article. Clinical Trial.
-
PD-L1 thresholds predict efficacy of immune checkpoint inhibition in first-line treatment of advanced gastroesophageal adenocarcinoma. A systematic review and meta-analysis of seven phase III randomized trials.ESMO Open. 2024 Nov;9(11):103967. doi: 10.1016/j.esmoop.2024.103967. Epub 2024 Nov 13. ESMO Open. 2024. PMID: 39541621 Free PMC article.
-
Comparison of different predictive biomarker testing assays for PD-1/PD-L1 checkpoint inhibitors response: a systematic review and network meta-analysis.Front Immunol. 2023 Sep 26;14:1265202. doi: 10.3389/fimmu.2023.1265202. eCollection 2023. Front Immunol. 2023. PMID: 37822932 Free PMC article.
References
-
- Morgan E., Soerjomataram I., Rumgay H., Coleman H.G., Thrift A.P., Vignat J. The global landscape of esophageal squamous cell carcinoma and esophageal adenocarcinoma incidence and mortality in 2020 and projections to 2040: new estimates from GLOBOCAN 2020. Gastroenterology. 2022;163(3):649. doi: 10.1053/j.gastro.2022.05.054. -58.e2. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

