Phase II study of rucaparib and nivolumab in patients with leiomyosarcoma
- PMID: 40514070
- PMCID: PMC12164637
- DOI: 10.1136/jitc-2025-012020
Phase II study of rucaparib and nivolumab in patients with leiomyosarcoma
Abstract
Background: Objective responses to immune checkpoint inhibitors (ICI) in leiomyosarcoma (LMS) are rare. Response rates may be increased by combination with other drugs known to promote immune infiltration, such as poly(ADP-ribose) polymerase (PARP) inhibitors, which have led to benefit in BRCA-altered uterine LMS. We therefore evaluated the combination of a PARP inhibitor, rucaparib, and the anti-programmed death receptor-1 monoclonal antibody, nivolumab, in patients with advanced LMS and investigated its effects on the tumor immune microenvironment.
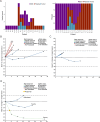
Methods: This was an open-label, single-center, single-arm, phase II study in patients with advanced refractory LMS. Full protocol available Patients were treated with rucaparib 600 mg orally, two times daily, continuously and nivolumab 480 mg intravenously on day 1 of a 28-day cycle. Re-staging scans were performed every 8 weeks. Blood and tissue samples were collected at baseline and at week 8 on treatment. The primary objective was the best objective response rate by 24 weeks using Response Evaluation Criteria in Solid Tumour (RECIST V.1.1). Secondary objectives included treatment-related toxicity, progression-free survival, overall survival, and changes in immune pathways in blood and tumor.
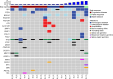
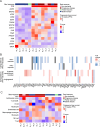
Results: 20 patients with LMS were enrolled. There was one partial response (PR) (5%) in a patient with uterine LMS and a somatic BRCA deep deletion. 19 (95%) patients had a treatment-related adverse event (TRAE) and 7 (35%) had a grade 3 or higher TRAE. Interferon (IFN) α and γ hallmark pathways were more highly expressed in patients who derived benefit from treatment (at least stable disease by 16 weeks) vs those who did not in both baseline (adjusted p=0.005 for IFN-α, 0.03 for IFN-γ) and on-treatment biopsies (adjusted p=0.0002 for IFN-α, 0.0001 for IFN-γ), but the abundance of tumor immune cell populations did not differ between these groups at either time point.
Conclusion: The addition of a PARP inhibitor did not improve the efficacy of ICI in LMS. Adverse events, especially due to overlapping toxicities, were frequent and often led to dose delays and modifications.
Keywords: Immune Checkpoint Inhibitor; Solid tumor.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: SM has provided consulting/advisory services for Deciphera and received research funding for clinical trials via the institution from Ascentage Pharma, Clovis, Bristol Myers Squibb, Jazz, Pfizer/Trillium, PTC Therapeutics, and Hutchinson Medipharma. ER has received funding for clinical trials via the institution from Iovance Biotherapeutics, Incyte Corporation, and Arcus Biosciences and reports stock ownership from Iovance Biotherapeutics and PMV Pharma. CMK has provided consulting/advisory services for Deciphera, received funding for clinical trials via the institution from Merck, Amgen, Inhbrx, IXRx, Curadev, Regeneron, and Servier and has a spouse employed at Daichii Sankyo. MLH has provided consulting/advisory services for AADi and Targeted Oncology and has a spouse employed by Sanofi until Feb. 2024. JEC has received research funding from ONO. PC has provided consulting/advisory services for Deciphera, Ningbo NewBay Medical and has received funding for clinical trials via the institution from Pfizer/Array, Deciphera, and Ningbo NewBay Medical Technology. SD'A has provided consulting/advisory services for Ratio, Adaptimmune, GI Innovation, EMD Serono, Amgen, Nektar, Immune Design, GSK, Incyte, Immunocore, Aadi, Pfizer, Servier, and Rain Theraputics and has received funding for clinical trials via the institution from Incyte, Merck, EMD Serano, Nektar, and Deciphera. MAD has received funding for clinical trials via the institution from Eli Lilly, AADi, and Sumitomo. MMG has provided consulting/advisory services for Epizyme, Ayala Pharmaceuticals, Rain Therapeutics, AADi, Ikena Oncology, and Kura Oncology, honoraria from Medscape, Guidepoint Global, Med Learning Group, Research to Practice, Great Debates and Updates, GLG, OncLive/MJH Life Sciences, and MJH/PER, received funding for clinical trials via the institution from Ayala Pharmaceuticals, AADi, Athenex, Boehringer Ingelheim, Foghorn Therapeutics, Ikena Oncology, GlaxoSmithKline, Rain Oncology, Regeneron, SpringWorks Therapeutics, SERVIER, Tango Therapeutics, Kymera, Erasca, and Vivace Therapeutics, has patents/ royalties/other intellectual property from UpToDate and GODDESS PRO Desmoid Tumor, travel/accommodations/expenses from Epizyme, and uncompensated relationship with Foundation Medicine. RGM has provided consulting /advisory services for AADi, Boehringer Ingelheim, Deciphera, and Peel Therapeutics, received royalties from UpToDate, received honoraria from the American Society of Clinical Oncology, and has equity ownership/stock options in Peel Therapeutics. AG has provided consulting/advisory services for Merck, Menarini/Stemline, received funding for clinical trials via the institution from Aadi, Lilly, Merio BioPharma and grant support from ASCO. VM has received research funding via the institution from AstraZeneca, Bristol Myers Squibb, Clasi, Cullinan Oncology, DualityBio, Eisai, Faeth Therapeutics, Karyopharm Therapeutics, Merck, Takeda, and Zymeworks; received travel support from Eisai and Merck; and provided consulting/advisory services for Clovis Oncology, Cullinan Oncology, DualityBio, Eisai, Faeth Therapeutics, GlaxoSmithKline, Immunocore, iTeos Therapeutics, Karyopharm Therapeutics, Lilly, Merck, Mereo BioPharma, MorphoSys, MSD, Novartis, Regeneron, Sutro Biopharma, and Zymeworks. JE has provided consulting services for AstraZeneca. WT has provided consulting/advisory services for AADi, Abbisko, Amgen, AmMax Bio, Avacta, Bayer Pharmaceuticals, BioAtla, Boehringer Ingelheim, C4 Therapeutics, Certis Oncology, Cogent, Curadev, Daiichi Sankyo, Deciphera, Ikena, IMGT, Inhibrx, Innova, Ipsen, PharmaEssentia, Ratio, Recordati, Servier, Sonata, and Synox; owns stock in Certis Oncology; and co-founded and owns stock in Atropos Therapeutics. All other authors report no relevant conflicts of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
