Increased Neuronal Expression of the Early Endosomal Adaptor APPL1 Replicates Alzheimer's Disease-Related Endosomal and Synaptic Dysfunction with Cholinergic Neurodegeneration
- PMID: 40514243
- PMCID: PMC12268982
- DOI: 10.1523/JNEUROSCI.2331-24.2025
Increased Neuronal Expression of the Early Endosomal Adaptor APPL1 Replicates Alzheimer's Disease-Related Endosomal and Synaptic Dysfunction with Cholinergic Neurodegeneration
Abstract
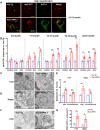
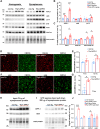
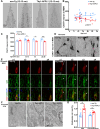
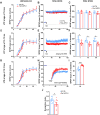
Endosomal system dysfunction within neurons is a prominent early feature of Alzheimer's disease (AD) pathology. Multiple AD risk factors are regulators of endocytosis and known to cause hyperactivity of the early endosome small GTPase rab5, resulting in neuronal endosomal pathway disruption and cholinergic neurodegeneration. Adaptor protein containing Pleckstrin homology domain, Phosphotyrosine binding domain, Leucine zipper motif (APPL1), an important rab5 effector protein and signaling molecule has been shown in vitro to interface between endosomal and neuronal dysfunction through a rab5-activating interaction with the BACE1-generated C-terminal fragment of amyloid precursor protein (APP-βCTF), a pathogenic APP fragment generated within endosomal compartments. To understand the contribution of APPL1 to AD-related endosomal dysfunction in vivo, we generated a transgenic mouse model overexpressing human APPL1 within neurons (Thy1-APPL1). Strongly supporting the important endosomal regulatory roles of APPL1 and their relevance to AD etiology, Thy1-APPL1 mice (both sexes) develop enlarged neuronal early endosomes and increased synaptic endocytosis due to increased rab5 activation. We demonstrated pathophysiological consequences of APPL1 overexpression, including functional changes in hippocampal long-term potentiation (LTP) and long-term depression (LTD), degeneration of large projection cholinergic neurons of the basal forebrain, and impaired hippocampal-dependent memory. Our evidence shows that neuronal APPL1 elevation modeling its functional increase in the AD brain induces a cascade of AD-related pathological effects within neurons, including early endosome anomalies, synaptic dysfunction, and selective neurodegeneration. Our in vivo model highlights the contributions of APPL1 to the pathobiology and neuronal consequences of early endosomal pathway disruption and its potential value as a therapeutic target.
Keywords: AD; APP-βCTF; APPL1; ChAT+ neuron; endosome; rab5.
Copyright © 2025 Jiang et al.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

Update of
-
Increased neuronal expression of the early endosomal adaptor APPL1 replicates Alzheimer's Disease-related endosomal and synaptic dysfunction with cholinergic neurodegeneration.bioRxiv [Preprint]. 2025 May 8:2024.09.19.613736. doi: 10.1101/2024.09.19.613736. bioRxiv. 2025. Update in: J Neurosci. 2025 Jul 16;45(29):e2331242025. doi: 10.1523/JNEUROSCI.2331-24.2025. PMID: 39345644 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
