Inhibitory effects of Levilactobacillus brevis IBRC-M10790 on apoptosis and inflammation induced by Clostridioides difficile culture supernatant in vitro
- PMID: 40514400
- PMCID: PMC12166082
- DOI: 10.1038/s41598-025-04975-5
Inhibitory effects of Levilactobacillus brevis IBRC-M10790 on apoptosis and inflammation induced by Clostridioides difficile culture supernatant in vitro
Abstract
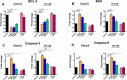
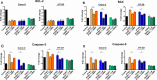
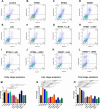
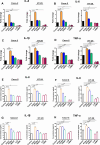
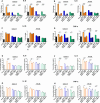
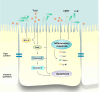
Clostridioides difficile infection (CDI) is a major cause of healthcare-associated diarrhea that contributes significantly to global morbidity and mortality. Bacterial virulence factors, mostly toxins, play key roles in CDI pathogenesis. Probiotic supplementation is a potential strategy to reduce the adverse effects of C. difficile and support intestinal homeostasis. This study aimed to investigate the inhibitory effects of live Levilactobacillus brevis IBRC-M10790 (LLB) and its membrane vesicles (LBMVs) on apoptosis and inflammation induced by released C. difficile virulence factors in vitro. We employed human colorectal adenocarcinoma Caco-2 and HT-29 cell lines, which are widely used as in vitro models against CDI. Viability and apoptosis of both cell lines were assessed using MTT and Annexin V/PI flow cytometry assays. Anti-inflammatory and anti-apoptotic effects of LLB and LBMVs were investigated following treatment with cell-free supernatants of toxigenic C. difficile RT001 (Tox-S), as well as the culture filtrates of non-toxigenic C. difficile RT084 and ATCC 700057 strains. The expression of apoptosis-related genes (BAX, BCL-2, Caspase-3, Caspase-9) and inflammatory markers (IL-6, IL-8, IL-1β, TNF-α) was measured by RT-qPCR, and cytokine production was analyzed by ELISA. C. difficile Tox-S and culture filtrate significantly reduced cell viability and increased the expression of apoptotic and proinflammatory markers in Caco-2 and HT-29 cells. LLB and LBMVs effectively modulated cell viability, reduced apoptosis, and downregulated the expression and production of inflammatory cytokines in both cell lines after exposure to C. difficile culture supernatants. These findings suggest that LLB and LBMVs could be exploited as potential supplement to the current treatment strategies against C. difficile-induced cellular injury and inflammation.
Keywords: Clostridioides difficile infection; Levilactobacillus brevis; Apoptosis; Inflammation; Membrane vesicles; Tox-S.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval: This work does not contain any studies related to human participants or animals. The study was approved by the Institutional Review Board of the Research Institute for Gastroenterology and Liver Diseases, Shahid Beheshti University of Medical Sciences, Tehran, Iran (Project No. IR.SBMU.RIGLD.REC.1395.211).
Figures
Similar articles
-
Curcumin and capsaicin regulate apoptosis and alleviate intestinal inflammation induced by Clostridioides difficile in vitro.Ann Clin Microbiol Antimicrob. 2022 Sep 26;21(1):41. doi: 10.1186/s12941-022-00533-3. Ann Clin Microbiol Antimicrob. 2022. PMID: 36155114 Free PMC article.
-
The anti-inflammatory and anti-apoptotic effects of Achillea millefolium L. extracts on Clostridioides difficile ribotype 001 in human intestinal epithelial cells.BMC Complement Med Ther. 2024 Jan 13;24(1):37. doi: 10.1186/s12906-024-04335-2. BMC Complement Med Ther. 2024. PMID: 38218845 Free PMC article.
-
Pasteurized form of a potential probiotic lactobacillus brevis IBRC-M10790 exerts anti-inflammatory effects on inflammatory bowel disease in vitro.BMC Complement Med Ther. 2024 Jul 10;24(1):258. doi: 10.1186/s12906-024-04576-1. BMC Complement Med Ther. 2024. PMID: 38987744 Free PMC article.
-
Cyclic diguanylate differentially regulates the expression of virulence factors and pathogenesis-related phenotypes in Clostridioides difficile.Microbiol Res. 2024 Sep;286:127811. doi: 10.1016/j.micres.2024.127811. Epub 2024 Jun 20. Microbiol Res. 2024. PMID: 38909416 Review.
-
The roles of host and pathogen factors and the innate immune response in the pathogenesis of Clostridium difficile infection.Mol Immunol. 2015 Feb;63(2):193-202. doi: 10.1016/j.molimm.2014.09.005. Epub 2014 Sep 18. Mol Immunol. 2015. PMID: 25242213 Free PMC article. Review.
References
-
- Ahmadi, A. et al. Molecular characterization and antimicrobial susceptibility of clostridioides difficile isolates in patients with inflammatory bowel disease in gorgan, Iran. Mol. Genet. Microbiol. Virol.39, 275–283 (2024).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

