Identifying Maternal Conditions Leading to Gabapentinoid Prescriptions in Pregnancy Using Electronic Health Records from Six European Countries: A Contribution from the IMI ConcePTION Project
- PMID: 40514582
- PMCID: PMC12515234
- DOI: 10.1007/s40264-025-01565-2
Identifying Maternal Conditions Leading to Gabapentinoid Prescriptions in Pregnancy Using Electronic Health Records from Six European Countries: A Contribution from the IMI ConcePTION Project
Abstract
Introduction and objective: Given the recent increase in the prescription and dispensation of gabapentinoids (gabapentin and pregabalin) and the importance of controlling for underlying maternal illnesses in drug safety studies, we aimed to develop algorithms for identifying maternal conditions leading to gabapentinoid prescribing among pregnant women using data from six electronic healthcare data sources across Europe.
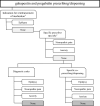
Methods: The study was conducted in Finland, France (Haute-Garonne), Italy (Emilia Romagna), Norway, Spain (Valencian region), and Wales (UK), covering three million pregnancies from 2006 to 2020. Algorithms were developed to detect epilepsy, neuropathic pain, and generalized anxiety disorder (GAD) (approved indications for gabapentinoids by the European Medicines Agency, with the exception of gabapentin for GAD) using data ± 1 year around the gabapentinoid prescription date. Data included prescriber specialty, primary and specialized health care diagnoses, and co-prescription/dispensation data. Additional analyses investigated potential unlicensed indications (such as fibromyalgia, restless legs syndrome, bipolar disorder) and potential for abuse (using codes for substance use disorders and alcohol withdrawal).
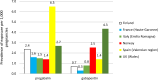
Results: Gabapentinoids were prescribed/dispensed in 1770 pregnancies (7.7 per 1000) in Spain, 2912 pregnancies (6.6 per 1000) in Wales, 3163 pregnancies (3.6 per 1000) in Norway, 2406 pregnancies (3.0 per 1000) in Finland, 908 pregnancies (2.2 per 1000) in Italy, and 269 pregnancies (1.9 per 1000) in France. A maternal condition related to gabapentinoid prescriptions was identified by the algorithm in 2797 (88.4%) in Norway, 2180 (74.9%) in Wales, 1269 (71.7%) in Spain, 1534 (63.8%) in Finland, 163 (60.6%) in France, and 396 (43.6%) pregnancies in Italy. Anxiety (licensed or unlicensed) was the most commonly captured condition in Wales (70.5%), Spain (51.5%), Finland (42.0%), and Italy (26.2%), whereas neuropathic pain prevailed in Norway (76.9%) and France (49.8%). Epilepsy was the least frequent maternal condition leading to gabapentinoid prescriptions across all data sources (below 15% of all pregnancies). The relative preponderance of these conditions differed between pregabalin and gabapentin. Additionally, unlicensed indications were captured in 0% to 13% of pregnancies, depending on the data source. The analyses of potential for abuse showed that records of alcohol withdrawal and/or substance use disorders (within 1 year before and after the gabapentinoids prescription/dispensation date) were present in 3% of pregnancies in Italy and up to 23% in Wales.
Conclusions: Our study provides valuable insights into gabapentinoid use during pregnancy, with anxiety being the most common condition among pregnant women with gabapentinoid prescriptions in Finland, Italy, Spain, and Wales, whereas neuropathic pain predominated in France and Norway. Moreover, we found that between 3 and 23% of these pregnancies were associated with substance abuse, underscoring the need for careful prescribing of commonly abused medicines. The proposed methods for detecting maternal conditions leading to prescribing will facilitate accurate assessment of medication use and safety during pregnancy, whilst addressing confounding by indication.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Funding: Open access funding provided by Université de Toulouse. The ConcePTION project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No. 821520. This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation program and EFPIA. The research leading to these results was conducted as part of the ConcePTION consortium. This paper only reflects the personal views of the stated authors. Open access funding was enabled by Toulouse University Hospital. Conflict of interest: AG is an employee of Janssen Biologics B.V. and owns stock/stock options in Johnson & Johnson, of which Janssen is a subsidiary. XM has received financial support from Allergan-Abbvie, Amgen-Horizon, Aptyspharma, Biogen, BMS, Grünenthal, Lilly, Lundbeck, Teva, Merck-Serono, Novartis, Orion, Pfizer, Roche, Sanofi-Genzyme, and Sun Pharma and non-financial support from Dr Reddy’s and SOS Oxygène not related to the submitted work. JM is an employee of Pfizer Inc. and owns stock/stock options of Pfizer Inc. All other co-authors have no competing interests to disclose. Ethics approval: Finland: Ethical approval is not required for register-based studies. The Institutional Review Board at the Finnish Institute for Health and Welfare approved the study and waived the requirement for obtaining informed consent for the secondary use of health administrative data from study participants (THL/543/6.02.00/2021). Data were handled and stored in accordance with the General Data Protection Regulation. France: The EFEMERIS cohort was approved by the French Data Protection Authority on 7 April 2005 (authorization number 05-1140). This study was performed on anonymized patient data. The women included in the EFEMERIS database were informed of their inclusion and of the potential use of their anonymized data for research purposes. They could oppose the use of their data at any time. The women included in the EFEMERIS database know that their collected and anonymized data can be used for medical research purposes and can thus be published. The study was approved by the EFEMERIS steering group. Data were handled and stored in accordance with the General Data Protection Regulation. Italy: The study was approved by the local ethical committee (approval number 593/2023/Oss/UniFe). Data were handled and stored in accordance with the General Data Protection Regulation and in agreement with the Authority for Healthcare and Welfare, Emilia Romagna Regional Health Service, Bologna, Italy. Norway: The study was approved by the Regional Committee for Research Ethics in South-East Norway (approval number 85224) and by the Data Protection Officer at the University of Oslo (approval number 519858). Data were handled and stored in accordance with the General Data Protection Regulation. Spain (Valencian region): The study (code: IMI-IMN-2019-01) was classified as an Observational Post-authorization Study “Other designs” (EPA-OD) by the Spanish Medicines Agency (AEMPS), available at: https://sede.aemps.gob.es and approved by the Arnau de Vilanova Hospital’s Clinical Research Ethics Committee on 29 th January 2020, according to the Spanish regulations (approval number 1/2020). At a regional level following the national Personal Data Protection and guaranteeing digital rights (Law 3/2018), the study was approved by the Commission of the Regional Government (PROSIGA), which has the right to give RDRU Fisabio authorization to process the data (references: SD2556; SD2577; SD2578; SD2579; SD2580; SD2581; SD2582). Wales: This study uses anonymized data held in the Secure Anonymised Information Linkage (SAIL) Databank. The SAIL Databank independent Information Governance Review Panel (IGRP) approved the study as part of project 0823, on 16 th October 2020. Consent to participate: As the data were anonymized, informed consent was not required. Consent for publication: Not applicable. Code availability: All code lists and scripts are available from the Zenodo repository [1]. Author contributions: The study was primarily conceived and designed by ABB and CDM. All authors reviewed and provided input on the study protocol. All authors reviewed the statistical analysis plan, assessed the feasibility of statistical analyses against the local data, and provided input for data source-specific tailoring. OP translated the statistical analysis plan, primarily written by ABB, into analysis script. All authors read and approved the final version. Finland: ML applied for the study approval and obtained the Finnish data in this study. VM and ML were responsible for the mapping of the Finnish data onto the ConcePTION CDM. VM was responsible for data curation, running scripts on the Finnish data and debugging. ML and MG contributed to data interpretation and benchmarking of the Finnish data in the study. ML reviewed the aggregated Finnish results and approved their upload to the DRE (safe server at UMC). France: CDM applied for the study approval and obtained the French data in this study. CDM and ABB were responsible for the mapping of the French data onto the ConcePTION CDM. ABB was responsible for data curation, running scripts on the French data and debugging. CDM, ABB, MB, JB contributed to data interpretation and benchmarking of the French data in the study. ABB reviewed the aggregated French results and approved their upload to the DRE (safe server at UMC). Spain (Valencian region): CCC obtained all required approvals—the Spanish Medicines Agency (AEMPS) classification and the Clinical Research Ethics Committee approval, and applied to the Regional Commission (PROSIGA) for the study data. LGV contributed to the reception and adequacy of the data format. LBB developed the mapping to the ConcePTION Common Data Model and executed the analysis scripts. During the script execution, LBB and CCC implemented the data quality assessment according to the study methodology and managed some issues during the process. LGV, CCC, and LBB contributed to data interpretation and the benchmarking of the Valencian region data and approved their upload to the DRE (safe server at UMC). CCC safeguarded custody of the local data into the institutional server. Wales: SJ applied for the study data and obtained all required approvals for the Wales data in this study. AC ran and cleaned the scripts. SJ curated and interpreted the data, benchmarked to published data, and approved uploading of aggregated data. Italy: EB and AN applied for the study data and obtained all required approvals for the Italian data in this study. AP was responsible for the mapping of the Italian data onto the ConcePTION CDM. MM was responsible for data curation, running scripts on the Italian data and debugging. AP and MM contributed to data interpretation and benchmarking of the Italian data in the study. MM reviewed the aggregated Italian results and approved their upload to the DRE (safe server at UMC). Norway: HN applied for the study data and obtained all required approvals for the Norwegian data in this study. HN was responsible for and HM and VRM contributed to data curation for the mapping of the Norwegian data onto the ConcePTION CDM. HN and VRM contributed to data interpretation and benchmarking of the Norwegian data in the study, and HN reviewed the aggregated Norwegian data and approved their upload to the DRE (safe server at UMC). Follow-up of data access providers and data analysis of aggregated data on the DRE was performed by ABB. The first draft of the manuscript was written by ABB and all authors commented on the previous versions of the manuscript. All authors contributed to the interpretation, discussed the results and approved the final version. Availability of data and material: All relevant data are within the paper and its supporting information files. Authors may not share the study data due to regulations, which restrict access and distribution to those with ethical and legal permission to use the data. The study material is available to other researchers upon an application to relevant register holders. The study protocol was registered in the EUPAS Registry (EUPAS43385) and is available from the Zenodo repository [2]. All code lists and scripts are available from the Zenodo repository [1].
Figures

References
-
- Paoletti O, RosaMGini. IMI-ConcePTION/DP1-Neuropathic-pain: DP1 ALGO final [Internet]. Zenodo; 2025. 10.5281/zenodo.14746934.
-
- Beau A-B, Damase-Michel C, Mo J, Moisset X. Final study protocols for demonstration projects submitted to EU PAS register (D1.3) [Internet]. Zenodo; 2022. 10.5281/zenodo.7476130.
-
- Johannessen Landmark C, Larsson PG, Rytter E, Johannessen SI. Antiepileptic drugs in epilepsy and other disorders—a population-based study of prescriptions. Epilepsy Res. 2009;87:31–9. - PubMed
-
- Wallach Kildemoes H, Hendriksen C, Andersen M. Drug utilization according to reason for prescribing: a pharmacoepidemiologic method based on an indication hierarchy. Pharmacoepidemiol Drug Saf. 2012;21:1027–35. - PubMed
-
- Charlton R, Damase-Michel C, Hurault-Delarue C, Gini R, Loane M, Pierini A, et al. Did advice on the prescription of sodium valproate reduce prescriptions to women? An observational study in three European countries between 2007 and 2016. Pharmacoepidemiol Drug Saf. 2019;28:1519–28. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

