Myelin-axon interface vulnerability in Alzheimer's disease revealed by subcellular proteomics and imaging of human and mouse brain
- PMID: 40514588
- PMCID: PMC12395420
- DOI: 10.1038/s41593-025-01973-8
Myelin-axon interface vulnerability in Alzheimer's disease revealed by subcellular proteomics and imaging of human and mouse brain
Abstract
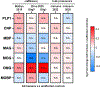
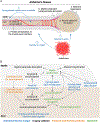
Myelin ensheathment is essential for rapid axonal conduction, metabolic support and neuronal plasticity. In Alzheimer's disease (AD), disruptions in myelin and axonal structures occur, although the underlying mechanisms remain unclear. We implemented proximity labeling subcellular proteomics of the myelin-axon interface in postmortem human brains from AD donors and 15-month-old male and female 5XFAD mice. We uncovered multiple dysregulated signaling pathways and ligand-receptor interactions, including those linked to amyloid-β processing, axonal outgrowth and lipid metabolism. Expansion microscopy confirmed the subcellular localization of top proteomic hits and revealed amyloid-β aggregation within the internodal periaxonal space and paranodal/juxtaparanodal channels. Although overall myelin coverage is preserved, we found reduced paranode density, aberrant myelination and altered paranode positioning around amyloid-plaque-associated dystrophic axons. These findings suggest that the myelin-axon interface is a critical site of protein aggregation and disrupted neuro-glial signaling in AD.
© 2025. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures
















References
-
- Lubetzki C, Sol-Foulon N, and Desmazieres A, Nodes of Ranvier during development and repair in the CNS. Nat Rev Neurol, 2020. 16(8): p. 426–439. - PubMed
-
- Nave KA and Werner HB, Ensheathment and Myelination of Axons: Evolution of Glial Functions. Annu Rev Neurosci, 2021. 44: p. 197–219. - PubMed
-
- Monje M, Myelin Plasticity and Nervous System Function. Annu Rev Neurosci, 2018. 41: p. 61–76. - PubMed
Methods-only references
-
- Feng G, et al. , Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron, 2000. 28(1): p. 41–51. - PubMed
-
- Dhaunchak AS, et al. , A proteome map of axoglial specializations isolated and purified from human central nervous system. Glia, 2010. 58(16): p. 1949–60. - PubMed
Extended Data Figure Reference
-
- Voas MG, et al. , alphaII-spectrin is essential for assembly of the nodes of Ranvier in myelinated axons. Curr Biol, 2007. 17(6): p. 562–8. - PubMed
-
- Boyle ME, et al. , Contactin orchestrates assembly of the septate-like junctions at the paranode in myelinated peripheral nerve. Neuron, 2001. 30(2): p. 385–97. - PubMed
MeSH terms
Substances
Grants and funding
- P30 DA018343/DA/NIDA NIH HHS/United States
- R01NS111961/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- RF1 AG058257/AG/NIA NIH HHS/United States
- R01 NS115544/NS/NINDS NIH HHS/United States
- R01NS115544/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
LinkOut - more resources
Full Text Sources
Medical

