Safety and efficacy of retreatment with immune checkpoint inhibitors after severe immune-related adverse events
- PMID: 40515478
- PMCID: PMC12166119
- DOI: 10.1093/oncolo/oyaf120
Safety and efficacy of retreatment with immune checkpoint inhibitors after severe immune-related adverse events
Abstract
Background: While immune checkpoint inhibitors (ICIs) have revolutionized cancer treatment, they can trigger severe immune-related adverse events (irAEs). The safety and efficacy of ICI retreatment after severe irAEs remain poorly understood.
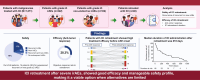
Methods: We conducted a retrospective analysis of 1271 patients with malignancies treated with ICIs at a university hospital in Japan between September 2014 and June 2023. We evaluated the incidence and characteristics of severe irAEs, defined as grade ≥3, and the safety and efficacy of ICI retreatment.
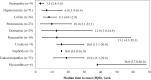
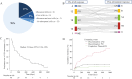
Results: Severe irAEs occurred in 222 patients (17.5%). Patients with single endocrinopathies were excluded, and 46 (28.4%) of the remaining 162 patients underwent ICI retreatment. Upon retreatment, 14 patients (30.4%) experienced recurrent or new grade ≥2 irAEs. One patient who experienced hepatotoxicity (grade 3) at initial ICI treatment developed a recurrence (grade 4). Regarding antitumor response, the objective response rate to retreatment was 28.3% (13/46), with 10.9% achieving complete and 17.4% partial response. The median duration of ICI administration after retreatment was 218 days (95% confidence interval [CI]: 84-399). At 1 year after retreatment, 15.4% (95% CI: 6.8-27.4) of patients discontinued due to irAEs, 44.4% (95% CI: 29.7-58.1) due to disease progression, 6.6% (95% CI: 1.7-16.3) completed planned treatment, and 33.4% (95% CI: 20.3-47.2) continued treatment.
Conclusions: ICI retreatment after severe irAEs demonstrated a manageable safety profile and promising efficacy, even in patients with grade ≥3 irAEs. ICI retreatment may be a viable option for patients with limited alternatives, particularly those showing favorable antitumor responses at initial treatment.
Keywords: efficacy; immune checkpoint inhibitors; immune-related adverse events; retreatment; safety.
© The Author(s) 2025. Published by Oxford University Press.
Conflict of interest statement
S.I. received personal fees from Ono Pharmaceutical Co., Ltd., Bristol-Myers Squibb Co., Ltd., Chugai Pharmaceutical Co., Ltd., MSD K.K., AstraZeneca Co., Ltd., and Merck Biopharma Co., Ltd. outside of the submitted work. T.H. received personal fees from AstraZeneca K.K., Takeda Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., Bristol-Myers Squibb Co., Ltd., Taiho Pharmaceutical Co., Ltd., MSD K.K., Merck Biopharma Co., Ltd., Pfizer Inc., and Eli Lilly Japan K.K., and grants from Novartis Pharma K.K., AstraZeneca K.K., BeiGene Inc., AbbVie Inc., Amgen Co., Ltd., and Chugai Pharmaceutical Co., Ltd., outside of the submitted work. T.M. received personal fees from AstraZeneca K.K. outside of the submitted work. M.I. received research funding from Nippon Boehringer Ingelheim Co., Ltd. and lecture fees from AstraZeneca K.K. and Shionogi Co., Ltd. outside of the submitted work. H.A. received grants from Ono Pharmaceutical Co., Ltd., MSD K.K., and Chugai Pharmaceutical Co., Ltd., and personal fees from Ono Pharmaceutical Co., Ltd., Bristol-Myers Squibb Co., Ltd., and MSD K.K. outside of the submitted work. Y.A. received grants and personal fees from Chugai Pharmaceutical Co., Ltd., and personal fees from Ono Pharmaceutical Co., Ltd., MSD K.K., and AstraZeneca K.K., outside of the submitted work. Y.A. is an Editorial Board member of Cancer Science.
Figures

References
-
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. N Engl J Med. 2015;373:123-135. https://doi.org/ 10.1056/NEJMoa1504627 - DOI - PMC - PubMed
-
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320-330. https://doi.org/ 10.1056/NEJMoa1412082 - DOI - PubMed
-
- André T, Shiu K-K, Kim TW, et al. ; KEYNOTE-177 Investigators. Pembrolizumab in microsatellite-instability–high advanced colorectal cancer. N Engl J Med. 2020;383:2207-2218. https://doi.org/ 10.1056/NEJMoa2017699 - DOI - PubMed
-
- Ishida Y, Agata Y, Shibahara K, Honjo T.. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887-3895. https://doi.org/ 10.1002/j.1460-2075.1992.tb05481.x - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

