Approaches to Invasive Fungal Diseases in Paediatric Cancer Centres: An Analysis of Current Practices and Challenges in Germany, Austria and Switzerland
- PMID: 40515497
- PMCID: PMC12166348
- DOI: 10.1111/myc.70074
Approaches to Invasive Fungal Diseases in Paediatric Cancer Centres: An Analysis of Current Practices and Challenges in Germany, Austria and Switzerland
Abstract
Background: Invasive fungal diseases (IFD) pose significant challenges in paediatric oncology. Their management is complicated by limited paediatric-specific evidence, lack of standardised protocols and variability in resources across centres. This study assessed current practices and addressed the challenges in the prevention, diagnosis and treatment of IFDs in paediatric oncology centres across Germany, Austria and Switzerland.
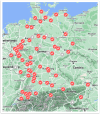
Methods: A questionnaire was distributed to senior paediatric oncologists in 70 paediatric oncology centres across Germany, Austria and Switzerland, gathering data on centre infrastructure, infectious disease (ID) expertise, annual cumulative IFD incidence in 2023, diagnostic tools, antifungal prophylaxis, treatment and follow-up practices for IFD. Responses were analysed descriptively.
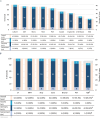
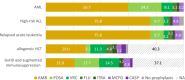
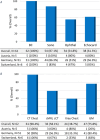
Results: Sixty-two centres responded, with a median of 56 (IQR 40-75) new oncological diagnoses per centre; 54.8% of centres managed allogeneic HCT patients. IFDs were reported in 88.7% of centres, with a median cumulative IFD incidence of 4.6% (IQR 3.0%-5.9%). No significant association was found between cumulative IFD incidence and the number of transplants, antifungal prophylaxis protocols and availability of ID consultation services. ID consultation was available in 58.1% of centres, with 24/7 support provided in 41.7% of these centres. Larger centres more frequently had paediatric ID specialists, ID consultation services and access to therapeutic drug monitoring.
Conclusions: The observed heterogeneity in mycology expertise and IFD management strategies across centres reflects the inherent complexity of IFDs and the diagnostic and therapeutic uncertainties amid limited evidence. Strengthening oncology-ID networks and implementing digital consultation platforms may promote high-quality, equitable care, particularly for those with fewer in-house resources.
Keywords: antifungal stewardship; aspergillosis; cancer; candidaemia; children; diagnosis; management; mycoses; prophylaxis; treatment.
© 2025 The Author(s). Mycoses published by Wiley‐VCH GmbH.
Conflict of interest statement
A.H.G. has received grants from Gilead, Merck, Sharp & Dohme and Pfizer and has served as a consultant to Amplyx, Astellas, Basilea, F2G, Gilead, Merck, Sharp & Dohme, Mundipharma, Pfizer and Scynexis. O.A.C. reports grants or contracts from iMi, iHi, DFG, BMBF, Cidara, DZIF, EU‐DG RTD, F2G, Gilead, MedPace, MSD, Mundipharma, Octapharma, Pfizer, Scynexis; consulting fees from Abbvie, AiCuris, Basilea, Biocon, Boston Strategic Partners, Cidara, Elion Therapeutics, Gilead, GSK, IQVIA, Janssen, Matinas, MedPace, Menarini, Melinta, Molecular Partners, MSG‐ERC, Mundipharma, Noxxon, Octapharma, Pardes, Partner Therapeutics, Pfizer, PSI, Scynexis, Seres, Seqirus, Shionogi, The Prime Meridian Group; speaker and lecture honoraria from Abbott, Abbvie, Akademie für Infektionsmedizin, Al‐Jazeera Pharmaceuticals/Hikma, amedes, AstraZeneca, Deutscher Ärzteverlag, Gilead, GSK, Grupo Biotoscana/United Medical/Knight, Ipsen Pharma, Medscape/WebMD, MedUpdate, MSD, Moderna, Mundipharma, Noscendo, Paul‐Martini‐Stiftung, Pfizer, Sandoz, Seqirus, Shionogi, streamedup!, Touch Independent, Vitis; payment for expert testimony from Cidara; participation on a DRC, DSMB, DMC, Advisory Board for AstraZeneca, Cidara, IQVIA, Janssen, MedPace, Melinta, PSI, Pulmocide, Vedanta Biosciences. Other authors had no conflicts of interest. T.N. has received authorship fees from uptodate.com (Wellesley, Massachusetts, USA) and reimbursement of travel expenses during consultancy work for the European Medicines Agency (EMA), steering committees of the PENTA Paediatric European Network for Treatment of AIDS (Padua, Italy), the Juvenile Inflammatory Cohort (JIR) (Lausanne, Switzerland). U.T. is funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—Project number 501830041. T.L. served as a consultant to Gilead Sciences, Pfizer, Merck/MSD, Mundipharma, Roche, Recordati, GlaxoSmithKline and Pharming, and in the speaker's bureau of Gilead Sciences, Merck/MSD, AstraZeneca, Recordati, Pfizer, Mundipharma and Sanofi Pasteur. V.S. served as a consultant to Merck/MSD, Pfizer and SANOFI, and has received speaker honoraria from GlaxoSmithKline, Merck/MSD, Pfizer and SANOFI.
Figures

References
-
- Gerard R., Gabriel F., Accoceberry I., et al., “Is There Still a Place for Serum Galactomannan in the Diagnosis of Invasive Aspergillosis in Children at High Risk and Under Antifungal Prophylaxis?,” Mycoses 67, no. 7 (2024): e13764. - PubMed
-
- Hassan M., Andresen F., Le U. T., et al., “The Role of Surgery for Invasive Pulmonary Aspergillosis in Paediatric Hemato‐Oncology Patients‐Can We Better Define It?,” Mycoses 67, no. 7 (2024): e13763. - PubMed
-
- Groll A. H., Pana D., Lanternier F., et al., “8th European Conference on Infections in Leukaemia: 2020 Guidelines for the Diagnosis, Prevention, and Treatment of Invasive Fungal Diseases in Paediatric Patients With Cancer or Post‐Haematopoietic Cell Transplantation,” Lancet Oncology 22, no. 6 (2021): e254–e269. - PubMed
-
- Chen S. C., Sorrell T. C., Chang C. C., Paige E. K., Bryant P. A., and Slavin M. A., “Consensus Guidelines for the Treatment of Yeast Infections in the Haematology, Oncology and Intensive Care Setting, 2014,” Internal Medicine Journal 44, no. 12b (2014): 1315–1332. - PubMed
-
- Lehrnbecher T., “The Clinical Management of Invasive Mold Infection in Children With Cancer or Undergoing Hematopoietic Stem Cell Transplantation,” Expert Review of Anti‐Infective Therapy 17, no. 7 (2019): 489–499. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

