Brain-Specific Gata4 Downregulation in Greywick Female Mice Models the Metabolic Subtype of Polycystic Ovary Syndrome
- PMID: 40515561
- PMCID: PMC12166494
- DOI: 10.1096/fj.202401718RR
Brain-Specific Gata4 Downregulation in Greywick Female Mice Models the Metabolic Subtype of Polycystic Ovary Syndrome
Abstract
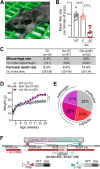
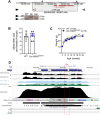
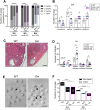
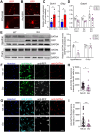
Polycystic ovary syndrome (PCOS) is a heterogenous disorder characterized by reproductive and metabolic abnormalities. PCOS etiology remains poorly understood, although the hypothalamus is suspected to play a central role in many cases. Human genetic studies have also shown an association with the transcription factor-coding gene GATA4, but without providing a functional link. Here, we show that adult Greywick female mice may bridge this gap. These mice phenocopy PCOS with partial penetrance, due to the serendipitous insertion of a Gata4 promoter-driven transgene in a strong enhancer region. Resulting robust transgene expression in subsets of hypothalamic neurons and glia impairs endogenous Gata4 expression, resulting in misexpression of genes linked to the control of fertility and food intake. We also show that this previously overlooked role of GATA4 in the hypothalamus can be replicated by conditional knockout approaches. Overall, this study sheds light not only on PCOS etiology but also on the role played by GATA4 in the central control of reproduction.
Keywords: GATA4; fertility; hypothalamus; metabolism; mouse model; polycystic ovary syndrome.
© 2025 The Author(s). The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Escobar‐Morreale H. F., “Polycystic Ovary Syndrome: Definition, Aetiology, Diagnosis and Treatment,” Nature Reviews. Endocrinology 14 (2018): 270–284. - PubMed
-
- Stener‐Victorin E., Teede H., Norman R. J., et al., “Polycystic Ovary Syndrome,” Nature Reviews. Disease Primers 10 (2024): 27. - PubMed
-
- Ruddenklau A. and Campbell R. E., “Neuroendocrine Impairments of Polycystic Ovary Syndrome,” Endocrinology 160 (2019): 2230–2242. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

