Mutant p53-specific CD8TCR-therapy combined with a CD4TCR prevents relapse of cancer and outgrowth of micrometastases
- PMID: 40515594
- PMCID: PMC12169035
- DOI: 10.1080/2162402X.2025.2514041
Mutant p53-specific CD8TCR-therapy combined with a CD4TCR prevents relapse of cancer and outgrowth of micrometastases
Abstract
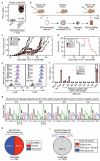
Relapse remains challenging in the treatment of metastatic cancers. More than 50% of human cancers harbor mutant p53 (mp53) as a cancer-specific target. We present the spontaneously metastasizing tumor model Ag104A to advance mp53-specific T cell receptor engineered T cell therapy (TCR-therapy). We identified in Ag104A an autochthonous p53D256E mutation as neoantigen recognized by a TCR isolated from CD8+ T cells (CD8TCR). Cloning of the Ag104A cancer revealed mp53 expression in >99% of cancer cells. Targeting mp53 by CD8TCR-therapy was initially therapeutic, but tumors escaped as cancer cells with reduced or lack of antigen expression. Therefore, we determined whether escape could be prevented by combining the mp53-specific CD8TCR with a CD4+ T cell-derived TCR (CD4TCR) recognizing a mutant antigen presented on the stroma of the cancer. No relapse occurred when the mp53-specific CD8TCR was combined with the stroma-recognizing CD4TCR. The combination therapy also prevented the development of macrometastases from cancer cells that had already spread to the lung at the time of TCR-therapy. Macrometastases were only observed after monotherapy. Thus, in a spontaneously metastatic model, tumor relapse and development of macrometastases can be prevented by combining a CD8TCR targeting an autochthonous p53-mutation with a mutation-specific CD4TCR recognizing tumor stroma.
Keywords: Adoptive cell transfer; TCR-therapy; metastatic tumor model; mutant p53; neoantigen.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



References
-
- Parkhurst MR, Robbins PF, Tran E, Prickett TD, Gartner JJ, Jia L, Ivey G, Li YF, El-Gamil M, Lalani A, et al. Unique neoantigens arise from somatic mutations in patients with gastrointestinal cancers. Cancer Discov. 2019;9(8):1022–1035. doi: 10.1158/2159-8290.CD-18-1494. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
