Melioidosis molecular diagnostics: An update
- PMID: 40515758
- PMCID: PMC12169043
- DOI: 10.1080/21505594.2025.2505698
Melioidosis molecular diagnostics: An update
Abstract
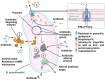
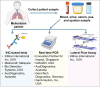
Melioidosis, a fatal tropical disease, presents a wide array of clinical manifestations, including abscesses, pneumonia, septic shock, bacteraemia, osteomyelitis, septic arthritis, and skin infection. The Centers for Disease Control and Prevention (CDC) has classified Burkholderia pseudomallei (B. pseudomallei), a gram-negative bacterium found in soil, as a Tier 1 select agent. Referred to as the "great mimicker," this organism can infect several organs imitating the symptoms of different illnesses. According to worldwide data, there are around 165,000 cases and 89,000 deaths annually. Current diagnostic procedures rely primarily on culturing B. pseudomallei, are slow and have low sensitivity, resulting in delayed treatment and higher fatality rates. This review examines the substantial difficulties related to diagnosing melioidosis in response to the urgent need for precise and prompt diagnosis. We have summarized the results of diagnostic kits that are currently sold in the market and assessed the market for melioidosis diagnostic kits.
Keywords: Melioidosis; artificial intelligence; diagnostics; early detection; liquid biopsy; point-of-care-test.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
