Transplants foster B cell alloimmunity by relaying extracellular vesicles to follicular dendritic cells
- PMID: 40516053
- PMCID: PMC12281710
- DOI: 10.1016/j.celrep.2025.115832
Transplants foster B cell alloimmunity by relaying extracellular vesicles to follicular dendritic cells
Abstract
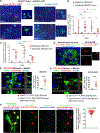
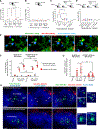
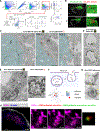
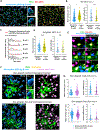
B cells play fundamental roles in transplant rejection. However, how allogeneic (allo)-antigens (Ags) are transported from allografts to follicular dendritic cells (FDCs) in lymphoid tissues for development of B cell responses remains unknown. We demonstrated that graft allo-Ags are relayed to FDCs via small extracellular vesicles (sEVs), which activate complement via immunoglobulin M (IgM) bound to vesicle phospholipids. Complement-opsonized allo-sEVs bind splenic marginal-zone B cells that shuttle the vesicles to FDCs, which retain and recycle the allo-sEVs so they are recognized by B cells. Accordingly, graft release of allo-sEVs promoted allo-major histocompatibility complex (MHC) accumulation in FDCs, germinal center formation, Ig switch and affinity maturation, and donor-specific antibodies, which decreased in allografts with impaired sEV secretion or when allo-Ags were delivered via disrupted sEVs. Importantly, human spleen FDCs bound allo-sEVs opsonized with human serum bearing active complement. Our findings provide insight into the mechanisms that lead to antibody-mediated rejection, for which there are no FDA-approved therapies.
Keywords: ABMR; CP: Immunology; allorecognition; antibody-mediated rejection; complement; donor-specific antibodies; exosomes; extracellular vesicles; follicular dendritic cells; natural antibodies; transplant rejection.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

Similar articles
-
WDFY1-expressing follicular dendritic cells play a critical role in lupus development in cGVHD mouse model.J Immunol. 2025 Jun 1;214(6):1160-1172. doi: 10.1093/jimmun/vkaf017. J Immunol. 2025. PMID: 40169152
-
Marginal zone B cells transport and deposit IgM-containing immune complexes onto follicular dendritic cells.Int Immunol. 2004 Oct;16(10):1411-22. doi: 10.1093/intimm/dxh142. Epub 2004 Aug 23. Int Immunol. 2004. PMID: 15326094
-
Single vesicle analysis reveals the release of tetraspanin positive extracellular vesicles into circulation with high intensity intermittent exercise.J Physiol. 2023 Nov;601(22):5093-5106. doi: 10.1113/JP284047. Epub 2023 Mar 16. J Physiol. 2023. PMID: 36855276 Free PMC article.
-
Polyclonal and monoclonal antibodies for treating acute rejection episodes in kidney transplant recipients.Cochrane Database Syst Rev. 2017 Jul 20;7(7):CD004756. doi: 10.1002/14651858.CD004756.pub4. Cochrane Database Syst Rev. 2017. PMID: 28731207 Free PMC article.
-
Sex and gender as predictors for allograft and patient-relevant outcomes after kidney transplantation.Cochrane Database Syst Rev. 2024 Dec 19;12(12):CD014966. doi: 10.1002/14651858.CD014966.pub2. Cochrane Database Syst Rev. 2024. PMID: 39698949
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

