UV RESISTANCE LOCUS 8 signalling enhances photosynthetic resilience to herbicide-induced damage in Arabidopsis thaliana
- PMID: 40516058
- PMCID: PMC12267928
- DOI: 10.1111/nph.70303
UV RESISTANCE LOCUS 8 signalling enhances photosynthetic resilience to herbicide-induced damage in Arabidopsis thaliana
Abstract
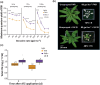
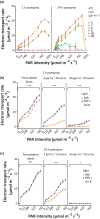
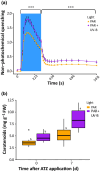
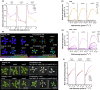
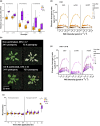
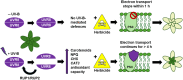
Perception of low irradiance ultraviolet B (UV-B) light (280-315 nm) by the UV RESISTANCE LOCUS 8 (UVR8) photoreceptor initiates signalling pathways that enhance plant defences to UV-B damage, mitigating the effects of higher photon irradiances. We therefore questioned whether UVR8 signalling could also prime plants against herbicide-induced damage, promoting postspray survival. We assessed the effects of a 2 d, low irradiance UV-B pretreatment on the photosynthetic resilience and survival of Arabidopsis thaliana plants treated with herbicides promoting photosynthetic disruption and oxidative stress. UV-B acclimation increased leaf carotenoid production, antioxidant activity and nonphotochemical quenching (NPQ) and delayed herbicide-induced reductions in electron transport rate (ETR), facilitating postspray regrowth and enhancing plant survival. In the absence of UV-B, this protection declined within 4 d, suggesting that it is unlikely to result from structural modifications. UV-B-mediated enhancement of photosynthetic resilience was abolished in the uvr8-6 mutant and increased in the UV-B hyper-responsive repressor of UV-B photomorphogenesis1/2 (rup1rup2) mutant, highlighting the involvement of UVR8 signalling. UV-B filtering during daylight acclimation also increased herbicide efficacy in Chenopodium, suggesting similar responses in agricultural weeds. UV-B-induced photoprotection enhances the resilience of plant photosystems to herbicide damage, providing a key target for increasing product efficacy and reducing usage.
Keywords: Arabidopsis; Chenopodium; UVR8; UV‐B; herbicide; photosynthesis.
© 2025 The Author(s). New Phytologist © 2025 New Phytologist Foundation.
Conflict of interest statement
None declared.
Figures
Similar articles
-
Protection of Photosynthesis by UVR8 and Cryptochromes in Arabidopsis Under Blue and UV Radiation.Plant Cell Environ. 2025 Aug;48(8):6321-6335. doi: 10.1111/pce.15608. Epub 2025 May 11. Plant Cell Environ. 2025. PMID: 40350778 Free PMC article.
-
Multiple roles for UV RESISTANCE LOCUS8 in regulating gene expression and metabolite accumulation in Arabidopsis under solar ultraviolet radiation.Plant Physiol. 2013 Feb;161(2):744-59. doi: 10.1104/pp.112.211375. Epub 2012 Dec 18. Plant Physiol. 2013. PMID: 23250626 Free PMC article.
-
Similar but different: unveiling the roles of atypical E2Fd/DEL2 and E2Ff/DEL3 transcription factors during the DNA damage response after UV-B exposure in Arabidopsis.Plant J. 2025 Jul;123(1):e70348. doi: 10.1111/tpj.70348. Plant J. 2025. PMID: 40644594
-
UVR8 mediated plant protective responses under low UV-B radiation leading to photosynthetic acclimation.J Photochem Photobiol B. 2014 Aug;137:67-76. doi: 10.1016/j.jphotobiol.2014.03.026. Epub 2014 Apr 13. J Photochem Photobiol B. 2014. PMID: 24780386 Review.
-
Antioxidants for female subfertility.Cochrane Database Syst Rev. 2013 Aug 5;(8):CD007807. doi: 10.1002/14651858.CD007807.pub2. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2017 Jul 28;7:CD007807. doi: 10.1002/14651858.CD007807.pub3. PMID: 23913583 Updated.
References
-
- Allorent G, Lefebvre‐Legendre L, Chappuis R, Kuntz M, Truong TB, Niyogi KK, Ulm R, Goldschmidt‐Clermont M. 2016. UV‐B photoreceptor‐mediated protection of the photosynthetic machinery in Chlamydomonas reinhardtii . Proceedings of the National Academy of Sciences, USA 113: 14864–14869. - PMC - PubMed
-
- Baker NR. 2008. Chlorophyll fluorescence: a probe of photosynthesis in vivo . Annual Review of Plant Biology 59: 89–113. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

