Evaluation of Organoid-Derived Exosomal microRNA as Liquid Biopsy for Colorectal Cancer: A Multicenter Cross-Sectional Study
- PMID: 40516061
- PMCID: PMC12166945
- DOI: 10.1111/cts.70270
Evaluation of Organoid-Derived Exosomal microRNA as Liquid Biopsy for Colorectal Cancer: A Multicenter Cross-Sectional Study
Abstract
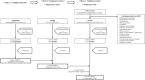
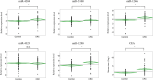
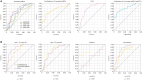
Exosomal microRNAs (miRNAs) are candidates for liquid biopsies. Organoid culture systems enable long-term expansion of the colon epithelium. This study evaluated exosomal miRNAs from colorectal cancer organoids for liquid biopsy. Organoids were established from normal colon and colorectal cancer tissues. Exosomes were isolated from conditioned media. miRNAs were extracted from exosomes and compared using microarray analysis. Exosomal miRNAs expression levels in the sera of healthy patients and patients with colorectal cancer were compared at a single institution. The multicenter study was validated using miRNAs upregulated in the serum of colorectal cancer patients, along with exosomal miRNAs reported to be upregulated in colorectal adenoma organoids and sera. A total of 44 exosomal miRNAs were commonly expressed in both normal colorectal epithelial cells and colorectal cancer organoids, whereas 59 were exclusively expressed in colorectal cancer organoids. In a single-center cohort study, two exosomal miRNAs (miR-4284 and miR-5100) were upregulated in the serum of colorectal cancer patients. In a multicenter study, four exosomal miRNAs (miR-4284, miR-5100, miR-1246, and miR-1290) were upregulated in the serum of patients with colorectal cancer. The combination of these four exosomal miRNAs had comparable diagnostic performance to carcinoembryonic antigen, with an area under the curve of 0.75 (95% confidence interval: 0.65-0.83) versus 0.79 (95% confidence interval: 0.70-0.87). Combining the four miRNAs with carcinoembryonic antigen improved diagnostic accuracy, with an area under the curve of 0.82 (95% confidence interval: 0.74-0.89). Exosomal miRNAs derived from colorectal cancer organoids can serve as diagnostic biomarkers for colorectal cancer.
Keywords: carcinoembryonic antigen; miR‐1246; miR‐1290; miR‐4284; miR‐5100.
© 2025 The Author(s). Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Liquid Biopsy for Colorectal Adenoma: Is the Exosomal miRNA Derived From Organoid a Potential Diagnostic Biomarker?Clin Transl Gastroenterol. 2021 May 12;12(5):e00356. doi: 10.14309/ctg.0000000000000356. Clin Transl Gastroenterol. 2021. PMID: 33979310 Free PMC article.
-
Exosomal tsRNA-Gly-5-0007 may be used as a diagnostic marker for colorectal cancer.Sci Rep. 2025 Jul 23;15(1):26751. doi: 10.1038/s41598-025-09830-1. Sci Rep. 2025. PMID: 40702001 Free PMC article.
-
Blood-based microRNAs as biomarkers for the diagnosis of colorectal cancer: a systematic review and meta-analysis.Br J Cancer. 2017 Mar 14;116(6):762-774. doi: 10.1038/bjc.2017.12. Epub 2017 Feb 2. Br J Cancer. 2017. PMID: 28152545 Free PMC article.
-
Serum exosomal hsa-let-7f-5p: A potential diagnostic biomarker for metastatic pancreatic cancer detection.World J Gastroenterol. 2025 Jul 14;31(26):109500. doi: 10.3748/wjg.v31.i26.109500. World J Gastroenterol. 2025. PMID: 40678708 Free PMC article.
-
The Predictive Value of Blood-Derived Exosomal miRNAs as Biomarkers in Breast Cancer: A Systematic Review.Clin Breast Cancer. 2025 Jan;25(1):e48-e55.e15. doi: 10.1016/j.clbc.2024.06.016. Epub 2024 Jul 6. Clin Breast Cancer. 2025. PMID: 39054208
Cited by
-
The 3D Language of Cancer: Communication via Extracellular Vesicles from Tumor Spheroids and Organoids.Int J Mol Sci. 2025 Jul 23;26(15):7104. doi: 10.3390/ijms26157104. Int J Mol Sci. 2025. PMID: 40806235 Free PMC article. Review.
References
-
- Keum N. and Giovannucci E., “Global Burden of Colorectal Cancer: Emerging Trends, Risk Factors and Prevention Strategies,” Nature Reviews. Gastroenterology & Hepatology 16 (2019): 713–732. - PubMed
-
- Lowenfels A. B., “Fecal Occult Blood Testing as a Screening Procedure for Colorectal Cancer,” Annals of Oncology 13 (2002): 40–43. - PubMed
-
- Bretthauer M., “Colorectal Cancer Screening,” Journal of Internal Medicine 270 (2011): 87–98. - PubMed
-
- Wu C.‐W. and Sung J. J.‐Y., “Colorectal Cancer Screening: Are Stool and Blood Based Tests Good Enough?,” Chinese Clinical Oncology 2 (2013): 8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

