Comparative effects of combustible cigarette versus electronic cigarette exposures on KRAS mutant lung cancer promotion
- PMID: 40516145
- PMCID: PMC12205659
- DOI: 10.1016/j.neo.2025.101185
Comparative effects of combustible cigarette versus electronic cigarette exposures on KRAS mutant lung cancer promotion
Abstract
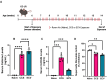
Despite the emerging public health concern related to the use of electronic cigarette vapors (ECV), its impact on lung cancer is poorly understood. We assessed the effect of ECV on lung tumorigenesis in a mouse model of lung adenocarcinoma. Mice were exposed to either room air, combustible cigarette smoke (CCS), or ECV 2 hours daily for 8 weeks at which lung samples were harvested and studied for different outcomes. We found that CCS, but not ECV, led to a significant increase in tumor burden. Immunophenotyping of both CCS- and ECV-exposed lungs displayed pronounced pro-tumor immunosuppressive phenotypes, characterized by significantly decreased CD4+ IFNγ+ and CD8+ GZMB+ T cells along with an elevated CD4+ FOXP3+ regulatory T cells. However, differential changes in myeloid cells were observed between CCS and ECV-exposed lungs. A microbiome profiling of matched stool and lung samples showed differences in the relative abundance of lung Pseudomonadotas, while gut Bacillota, particularly Turicibacter, and Ileibacterium were increased by CCS and ECV. We conclude that both CCS and ECV exposure under the applied regimen lead to a protumor immune suppressive lung microenvironment although with different magnitudes and slightly different phenotypes that might explain their differential effects on tumor burden warranting further studies.
Keywords: Cigarette smoke; Electronic cigarette; Kras; Lung cancer; Microbiome.
Copyright © 2025. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: SJM reports past funding from Arrowhead Pharma and Boehringer Ingelheim outside the submitted work.
Figures





References
-
- Ferlay J., Soerjomataram I., Dikshit R., Eser S., Mathers C., Rebelo M., et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer. 2015;136(5):E359–E386. - PubMed
-
- Mannino D.M., Aguayo S.M., Petty T.L., Redd S.C. Low lung function and incident lung cancer in the United States: data from the first national health and nutrition examination survey follow-up. Arch. Intern. Med. 2003;163(12):1475–1480. - PubMed
-
- Islami F., Marlow E.C., Thomson B., McCullough M.L., Rumgay H., Gapstur S.M., et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J. Clin. 2019:2024. - PubMed
-
- Youlden D.R., Cramb S.M., Baade P.D. The International Epidemiology of Lung Cancer: geographical distribution and secular trends. J. Thorac. Oncol. 2008;3(8):819–831. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

