Regulation of steady state ribosomal transcription in Mycobacterium tuberculosis: Intersection of sigma subunits, superhelicity, and transcription factors
- PMID: 40516872
- PMCID: PMC12312024
- DOI: 10.1016/j.jbc.2025.110369
Regulation of steady state ribosomal transcription in Mycobacterium tuberculosis: Intersection of sigma subunits, superhelicity, and transcription factors
Abstract
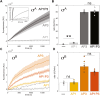
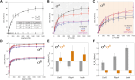
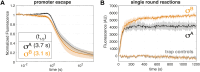
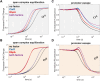
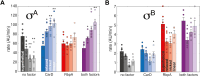
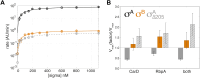
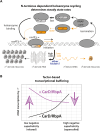
rRNA regulation in Mycobacterium tuberculosis (Mtb) is tightly linked to nutrient availability, growth phase, and global gene expression, influencing Mtb's adaptability and pathogenicity. Unlike most bacteria, Mtb has a single ribosomal operon with two promoters, rrnAP3 and rrnAP1, and a high ratio of sigma (σ) factors to genome size. While σA is the primary driver of ribosomal transcription, σB has been suggested to contribute under various conditions, though its role remains unclear. Here, we quantify steady-state transcription rates in reconstituted reactions and demonstrate that σA-driven transcription from rrnAP3 dominates rRNA production, with minimal contributions from σB or rrnAP1. Kinetic analysis suggests that σB holoenzymes exhibit slower DNA unwinding and holoenzyme recycling. We also show that transcription factors CarD and RbpA reverse and buffer, respectively, the stimulatory effects of negative superhelicity on σA-driven rRNA transcription. Finally, we identify the N-terminal 205 amino acids of σA as a key determinant of its increased activity relative to σB. Our findings reveal the intricate interplay of promoter sequence, σ factor identity, DNA superhelicity, and transcription factors in shaping transcription initiation kinetics to ultimately influence rRNA production in Mtb.
Keywords: CarD; DNA topology; Mycobacterium tuberculosis; RNA polymerase; RbpA; bacterial transcription; gene regulation; kinetics; rRNA; sigma factors; steady state; superhelicity; transcription factors.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures

Update of
-
Regulation of Steady State Ribosomal Transcription in Mycobacterium tuberculosis: Intersection of Sigma Subunits, Superhelicity, and Transcription Factors.bioRxiv [Preprint]. 2025 Feb 27:2025.02.24.639987. doi: 10.1101/2025.02.24.639987. bioRxiv. 2025. Update in: J Biol Chem. 2025 Jun 12;301(8):110369. doi: 10.1016/j.jbc.2025.110369. PMID: 40060575 Free PMC article. Updated. Preprint.
Similar articles
-
Regulation of Steady State Ribosomal Transcription in Mycobacterium tuberculosis: Intersection of Sigma Subunits, Superhelicity, and Transcription Factors.bioRxiv [Preprint]. 2025 Feb 27:2025.02.24.639987. doi: 10.1101/2025.02.24.639987. bioRxiv. 2025. Update in: J Biol Chem. 2025 Jun 12;301(8):110369. doi: 10.1016/j.jbc.2025.110369. PMID: 40060575 Free PMC article. Updated. Preprint.
-
Diverse impacts of different rpoB mutations on the anti-tuberculosis efficacy of capreomycin.EBioMedicine. 2025 Jul;117:105776. doi: 10.1016/j.ebiom.2025.105776. Epub 2025 May 30. EBioMedicine. 2025. PMID: 40449326 Free PMC article.
-
Aromatic Patch in WhiB-Like Transcription Factors Facilitates Primary Sigma Factor Interaction in Mycobacterium tuberculosis.bioRxiv [Preprint]. 2025 Jun 5:2025.06.05.653497. doi: 10.1101/2025.06.05.653497. bioRxiv. 2025. PMID: 40502120 Free PMC article. Preprint.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
-
- Global Tuberculosis Programme (GTB) World Health Organization; Geneva: 2024. WHO Global tuberculosis report 2024.
-
- Rodrigue S., Provvedi R., Jacques P.É., Gaudreau L., Manganelli R. The sigma factors of Mycobacterium tuberculosis. FEMS. Microbiol. Rev. 2006;30:926–941. - PubMed
LinkOut - more resources
Full Text Sources

