Large scale causal modeling to identify adults at risk for combined and common variable immunodeficiencies
- PMID: 40517144
- PMCID: PMC12167375
- DOI: 10.1038/s41746-025-01761-5
Large scale causal modeling to identify adults at risk for combined and common variable immunodeficiencies
Abstract
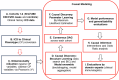
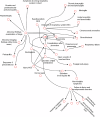
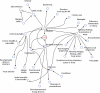
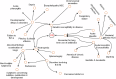
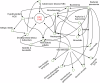
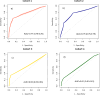
Combined immunodeficiencies (CID) and common variable immunodeficiencies (CVID), prevalent yet substantially underdiagnosed primary immunodeficiencies, necessitate improved early detection. Leveraging large-scale electronic health records (EHR) from four nationwide US cohorts, we developed a novel causal Bayesian Network (BN) model to identify antecedent clinical phenotypes associated with CID/CVID. Consensus directed acyclic graphs (DAGs) demonstrated robust predictive performance within each cohort (ROC AUC: 0.61-0.77) and generalizability across unseen cohorts (ROC AUC: 0.56-0.72) in identifying CID/CVID, despite varying inclusion criteria across cohorts. The consensus DAGs reveal causal relationships between comorbidities preceding CID/CVID diagnosis, including autoimmune and blood disorders, lymphomas, organ damage or inflammation, respiratory conditions, genetic anomalies, recurrent infections, and allergies. Further evaluation through causal inference and by expert clinical immunologists substantiates the clinical relevance of the identified phenotypic trajectories. These findings hold promise for translation into improved clinical practice, potentially leading to earlier identification and intervention of adults at risk for CID/CVID.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: G.P., K.B., N.V.V. and V.I. are full-time employees of Pfizer and hold stock/stock options. The other authors do not have any financial or non-financial competing interests to declare.
Figures
Similar articles
-
Large-scale deep learning analysis to identify adult patients at risk for combined and common variable immunodeficiencies.Commun Med (Lond). 2023 Dec 20;3(1):189. doi: 10.1038/s43856-023-00412-8. Commun Med (Lond). 2023. PMID: 38123736 Free PMC article.
-
CVID-Associated Tumors: Czech Nationwide Study Focused on Epidemiology, Immunology, and Genetic Background in a Cohort of Patients With CVID.Front Immunol. 2019 Jan 22;9:3135. doi: 10.3389/fimmu.2018.03135. eCollection 2018. Front Immunol. 2019. PMID: 30723478 Free PMC article.
-
Combined immunodeficiency (CVID and CD4 lymphopenia) is associated with a high risk of malignancy among adults with primary immune deficiency.Clin Exp Immunol. 2021 May;204(2):251-257. doi: 10.1111/cei.13579. Epub 2021 Feb 25. Clin Exp Immunol. 2021. PMID: 33497464 Free PMC article.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
[Genetic basis of common variable immunodeficiency: from common to variable].Ann Biol Clin (Paris). 2021 Oct 1;79(5):407-413. doi: 10.1684/abc.2021.1670. Ann Biol Clin (Paris). 2021. PMID: 34704938 Review. French.
References
-
- Bousfiha, A. et al. The 2022 Update of IUIS Phenotypical Classification for Human Inborn Errors of Immunity. J. Clin. Immunol.42, 1508–1520 (2022). - PubMed
LinkOut - more resources
Full Text Sources

