Cutaneous nerve fiber pathology and function in Parkinson's disease and atypical parkinsonism - a cohort study
- PMID: 40517154
- PMCID: PMC12167382
- DOI: 10.1038/s41531-025-01030-y
Cutaneous nerve fiber pathology and function in Parkinson's disease and atypical parkinsonism - a cohort study
Abstract
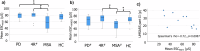
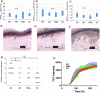
There is scientific evidence for ongoing neurodegeneration and alpha-synuclein pathology involving the peripheral nervous system in Parkinson's disease (PD) and multiple system atrophy (MSA). We explored putative disease-mirroring properties of cutaneous nerve fibers in patients with PD (n = 20), MSA (n = 12), four-repeat tauopathies (n = 11), and controls (n = 20). Assessments included clinical rating scales, blood sampling, sudomotor testing, skin punch biopsies from the neck and leg, and 1-year follow-up. Skin alpha-synuclein seeding amplification assay (SAA) and determination of intraepidermal nerve fiber density (IENFD) were performed. Reduced electrochemical skin conductance was evident in MSA, associated with clinical rating scores. Cervical skin SAA (PD vs controls) achieved a 100% sensitivity and 70% specificity for detecting PD. We found no difference in baseline IENFD, nor in 1-year changes, in patients relative to controls. Baseline IENFD, plasma neurofilament light, and SAA kinetics associated with 1-year clinical disease progression in MSA. Skin may harbor promising prognostic properties in MSA.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: M.A. declares no competing interest. W.P. has received funding from Parkinsonfonden Sweden. A.J.T. is funded by Novo Nordisk Borregaard Clinical Ascending grant (NNF23OC0082689) and has received honorarium from AstraZeneca UK limited, Novo Nordisk Denmark A/S, Alnylam Pharmaceuticals and Sanofi A/S.K.S. declares no competing interest. H.Z. is a Wallenberg Scholar and a Distinguished Professor at the Swedish Research Council supported by grants from the Swedish Research Council (#2023-00356; #2022-01018 and #2019-02397), the European Union’s Horizon Europe research and innovation programme under grant agreement No 101053962, Swedish State Support for Clinical Research (#ALFGBG-71320), the Alzheimer Drug Discovery Foundation (ADDF), USA (#201809-2016862), the AD Strategic Fund and the Alzheimer’s Association (#ADSF-21-831376-C, #ADSF-21-831381-C, #ADSF-21-831377-C, and #ADSF-24-1284328-C), the European Partnership on Metrology, co-financed from the European Union’s Horizon Europe Research and Innovation Programme and by the Participating States (NEuroBioStand, #22HLT07), the Bluefield Project, Cure Alzheimer’s Fund, the Olav Thon Foundation, the Erling-Persson Family Foundation, Familjen Rönströms Stiftelse, Stiftelsen för Gamla Tjänarinnor, Hjärnfonden, Sweden (#FO2022-0270), the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 860197 (MIRIADE), the European Union Joint Programme – Neurodegenerative Disease Research (JPND2021-00694), the National Institute for Health and Care Research University College London Hospitals Biomedical Research Centre, and the UK Dementia Research Institute at UCL (UKDRI-1003). H.Z. has served at scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZPath, Amylyx, Annexon, Apellis, Artery Therapeutics, AZTherapies, Cognito Therapeutics, CogRx, Denali, Eisai, LabCorp, Merry Life, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, has given lectures sponsored by Alzecure, Biogen, Cellectricon, Fujirebio, Lilly, Novo Nordisk, Roche, and WebMD, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). K.B. is supported by the Swedish Research Council (#2017-00915 and #2022-00732), the Swedish Alzheimer Foundation (#AF-930351, #AF-939721, #AF-968270, and #AF-994551), Hjärnfonden, Sweden (#FO2017-0243 and #ALZ2022-0006), the Swedish state under the agreement between the Swedish government and the County Councils, the ALF-agreement (#ALFGBG-715986 and #ALFGBG-965240), the European Union Joint Program for Neurodegenerative Disorders (JPND2019-466-236), the Alzheimer’s Association 2021 Zenith Award (ZEN-21-848495), the Alzheimer’s Association 2022-2025 Grant (SG-23-1038904 QC), La Fondation Recherche Alzheimer (FRA), Paris, France, the Kirsten and Freddy Johansen Foundation, Copenhagen, Denmark, and Familjen Rönströms Stiftelse, Stockholm, Sweden. KB has served as a consultant and at advisory boards for Abbvie, AC Immune, ALZPath, AriBio, BioArctic, Biogen, Eisai, Lilly, Moleac Pte. Ltd, Neurimmune, Novartis, Ono Pharma, Prothena, Roche Diagnostics, Sanofi and Siemens Healthineers; has served at data monitoring committees for Julius Clinical and Novartis; has given lectures, produced educational materials and participated in educational programs for AC Immune, Biogen, Celdara Medical, Eisai and Roche Diagnostics; and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program, outside the work presented in this paper. P.K. declares no competing interest. P.S. has received honorarium for lectures or scientific board consultations from Lundbeck, AstraZeneca, Bial, and AbbVie.
Figures
Similar articles
-
Alpha-synuclein oligomers and small nerve fiber pathology in skin are potential biomarkers of Parkinson's disease.NPJ Parkinsons Dis. 2021 Dec 20;7(1):119. doi: 10.1038/s41531-021-00262-y. NPJ Parkinsons Dis. 2021. PMID: 34930911 Free PMC article.
-
Clinical Utility of Skin Biopsy in Differentiating between Parkinson's Disease and Multiple System Atrophy.Parkinsons Dis. 2015;2015:167038. doi: 10.1155/2015/167038. Epub 2015 Apr 6. Parkinsons Dis. 2015. PMID: 25945280 Free PMC article.
-
Cutaneous α-Synuclein Signatures in Patients With Multiple System Atrophy and Parkinson Disease.Neurology. 2023 Apr 11;100(15):e1529-e1539. doi: 10.1212/WNL.0000000000206772. Epub 2023 Jan 19. Neurology. 2023. PMID: 36657992 Free PMC article.
-
Cerebrospinal fluid and blood neurofilament light chain in Parkinson's disease and atypical parkinsonian syndromes: a systematic review and Bayesian network meta-analysis.J Neurol. 2025 Apr 3;272(4):311. doi: 10.1007/s00415-025-13051-x. J Neurol. 2025. PMID: 40180649
-
Is Multiple System Atrophy a Prion-like Disorder?Int J Mol Sci. 2021 Sep 18;22(18):10093. doi: 10.3390/ijms221810093. Int J Mol Sci. 2021. PMID: 34576255 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

