Advances in molecular pathology and therapy of non-small cell lung cancer
- PMID: 40517166
- PMCID: PMC12167388
- DOI: 10.1038/s41392-025-02243-6
Advances in molecular pathology and therapy of non-small cell lung cancer
Abstract
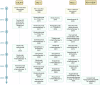
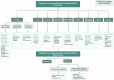
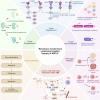
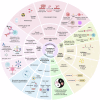
Over the past two decades, non-small cell lung cancer (NSCLC) has witnessed encouraging advancements in basic and clinical research. However, substantial unmet needs remain for patients worldwide, as drug resistance persists as an inevitable reality. Meanwhile, the journey towards amplifying the breadth and depth of the therapeutic effect requires comprehending and integrating diverse and profound progress. In this review, therefore, we aim to comprehensively present such progress that spans the various aspects of molecular pathology, encompassing elucidations of metastatic mechanisms, identification of therapeutic targets, and dissection of spatial omics. Additionally, we also highlight the numerous small molecule and antibody drugs, encompassing their application alone or in combination, across later-line, frontline, neoadjuvant or adjuvant settings. Then, we elaborate on drug resistance mechanisms, mainly involving targeted therapies and immunotherapies, revealed by our proposed theoretical models to clarify interactions between cancer cells and a variety of non-malignant cells, as well as almost all the biological regulatory pathways. Finally, we outline mechanistic perspectives to pursue innovative treatments of NSCLC, through leveraging artificial intelligence to incorporate the latest insights into the design of finely-tuned, biomarker-driven combination strategies. This review not only provides an overview of the various strategies of how to reshape available armamentarium, but also illustrates an example of clinical translation of how to develop novel targeted drugs, to revolutionize therapeutic landscape for NSCLC.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

