Changes in metabolite profiles in the cerebrospinal fluid and in human neuronal cells upon tick-borne encephalitis virus infection
- PMID: 40517242
- PMCID: PMC12166563
- DOI: 10.1186/s12974-025-03478-4
Changes in metabolite profiles in the cerebrospinal fluid and in human neuronal cells upon tick-borne encephalitis virus infection
Abstract
Background: Tick-borne encephalitis virus (TBEV) is a significant threat to human health. The virus causes potentially fatal disease of the central nervous system (CNS), for which no treatments are available. TBEV infected individuals display a wide spectrum of neuronal disease, the determinants of which are undefined. Changes to host metabolism and virus-induced immunity have been postulated to contribute to the neuronal damage observed in infected individuals. In this study, we evaluated the cytokine, chemokine, and metabolic alterations in the cerebrospinal fluid (CSF) of symptomatic patients infected with TBEV presenting with meningitis or encephalitis. Our aim was to investigate the host immune and metabolic responses associated with specific TBEV infectious outcomes.
Methods: CSF samples of patients with meningitis (n = 27) or encephalitis (n = 25) were obtained upon consent from individuals hospitalised with confirmed TBEV infection in Brno. CSF from uninfected control patients was also collected for comparison (n = 12). A multiplex bead-based system was used to measure the levels of pro-inflammatory cytokines and chemokines. Untargeted metabolomics followed by bioinformatics and integrative omics were used to profile the levels of metabolites in the CSF. Human motor neurons (hMNs) were differentiated from induced pluripotent stem cells (iPSCs) and infected with the highly pathogenic TBEV-Hypr strain to profile the role(s) of identified metabolites during the virus lifecycle. Virus infection was quantified via plaque assay.
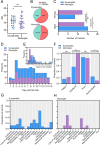
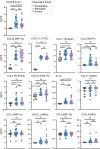
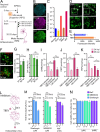
Results: Significant differences in proinflammatory cytokines (IFN-α2, TSLP, IL-1α, IL-1β, GM-CSF, IL-12p40, IL-15, and IL-18) and chemokines (IL-8, CCL20, and CXCL11) were detected between neurological-TBEV and control patients. A total of 32 CSF metabolites differed in TBE patients with meningitis and encephalitis. CSF S-Adenosylmethionine (SAM), Fructose 1,6-bisphosphate (FBP1) and Phosphoenolpyruvic acid (PEP) levels were 2.4-fold (range ≥ 2.3-≥3.2) higher in encephalitis patients compared to the meningitis group. CSF urocanic acid levels were significantly lower in patients with encephalitis compared to those with meningitis (p = 0.012209). Follow-up analyses showed fluctuations in the levels of O-phosphoethanolamine, succinic acid, and L-proline in the encephalitis group, and pyruvic acid in the meningitis group. TBEV-infection of hMNs increased the production of SAM, FBP1 and PEP in a time-dependent manner. Depletion of the metabolites with characterised pharmacological inhibitors led to a concentration-dependent attenuation of virus growth, validating the identified changes as key mediators of TBEV infection.
Conclusions: Our findings reveal that the neurological disease outcome of TBEV infection is associated with specific and dynamic metabolic signatures in the cerebrospinal fluid. We describe a new in vitro model for in-depth studies of TBEV-induced neuropathogenesis, in which the depletion of identified metabolites limits virus infection. Collectively, this reveals new biomarkers that can differentiate and predict TBEV-associated neurological disease. Additionally, we have identified novel therapeutic targets with the potential to significantly improve patient outcomes and deepen our understanding of TBEV pathogenesis.
Keywords: Cerebrospinal fluid; Chemokines; Human motor neurons; Metabolomics; Neuroinflammation; Pro-inflammatory cytokines; Tick-borne encephalitis virus.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval and consent to participate: Samples of CSF were obtained upon consent from individuals hospitalized with confirmed TBEV infection in České Budějovice, Czech Republic, under protocols approved by the ethical committees of the Hospital in České Budějovice (approval no. 103/19) and the Biology Centre of the Czech Academy of Sciences (approval no. 1/2018), and in Brno under protocol approved by the ethical committee of the University Hospital Brno, Czech Republic (date of approval: 27 June 2018). Storage and shipping were also be covered by the above ethical approvals. Clinical data were obtained at the treating hospitals. Disease severity was evaluated according to the following scale: mild, flu-like symptoms with meningeal irritation defined as meningitis, characterized by fever, fatigue, nausea, headache, back pain, arthralgia/myalgia and neck or back stiffness; moderate, previous symptoms together with tremor, vertigo, somnolence, and photophobia defined as meningoencephalitis; severe, prolonged neurological consequences including ataxia, titubation, altered mental status, memory loss, quantitative disturbance of consciousness, and palsy revealed as encephalitis, encephalomyelitis, or encephalomyeloradiculitis (Bogovic and Strle, 2015; Ruzek et al., 2019). Serological and molecular analysis were performed in line with “The Code of Ethics of the World Medical Association (Declaration of Helsinki)” and according to good clinical practice guidelines. In accordance with local legislation, no formal approval by a research ethics committee was required, as either anonymous or clinical samples were used for research purposes. Patients (or their parents) signed informed consent prior to sample collection. Samples were investigated anonymously. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures



Similar articles
-
Changes in cytokine and chemokine profiles in mouse serum and brain, and in human neural cells, upon tick-borne encephalitis virus infection.J Neuroinflammation. 2019 Nov 7;16(1):205. doi: 10.1186/s12974-019-1596-z. J Neuroinflammation. 2019. PMID: 31699097 Free PMC article.
-
Metabolomic Profiling of Cerebrospinal Fluid Reveals Metabolite Biomarkers in Tick-Borne Encephalitis Patient.J Med Virol. 2024 Nov;96(11):e70082. doi: 10.1002/jmv.70082. J Med Virol. 2024. PMID: 39569456 Free PMC article.
-
Integrative RNA profiling of TBEV-infected neurons and astrocytes reveals potential pathogenic effectors.Comput Struct Biotechnol J. 2022 May 30;20:2759-2777. doi: 10.1016/j.csbj.2022.05.052. eCollection 2022. Comput Struct Biotechnol J. 2022. PMID: 35685361 Free PMC article.
-
EAN consensus review on prevention, diagnosis and management of tick-borne encephalitis.Eur J Neurol. 2017 Oct;24(10):1214-e61. doi: 10.1111/ene.13356. Epub 2017 Aug 1. Eur J Neurol. 2017. PMID: 28762591 Review.
-
Cell-Mediated Immune Responses and Immunopathogenesis of Human Tick-Borne Encephalitis Virus-Infection.Front Immunol. 2018 Sep 26;9:2174. doi: 10.3389/fimmu.2018.02174. eCollection 2018. Front Immunol. 2018. PMID: 30319632 Free PMC article. Review.
References
-
- Gelpi E, Preusser M, Garzuly F, Holzmann H, Heinz FX, Budka H. Visualization of central European tick-borne encephalitis infection in fatal human cases. J Neuropathol Exp Neurol. 2005;64(6):506–12. - PubMed
-
- Bai Y, Xiao J, Moming A, Fu J, Wang J, Zhou M, Chen C, Shi J, Zhang J, Fan Z, et al. Identification and characterization of new Siberian subtype of tick-borne encephalitis virus isolates revealed genetic variations of the Chinese strains. Infect Genet Evol. 2024;124:105660. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

