Migration and invasion of cancer stem cells are prevented by low-intensity pulsed ultrasound therapy
- PMID: 40517246
- PMCID: PMC12166576
- DOI: 10.1186/s12935-025-03854-3
Migration and invasion of cancer stem cells are prevented by low-intensity pulsed ultrasound therapy
Abstract
Background: Ultrasound is considered a safe and non-invasive tool in regenerative medicine. In particular, low-intensity pulsed ultrasound (LIPUS) has been used in the clinic for more than twenty years for applications in bone healing. It has been demonstrated to be an effective tool to treat different chronic diseases. We sought to evaluate the effects produced by LIPUS on the properties of human breast cancer stem cells (bCSCs).
Methods: Cells were stimulated using a traditional ultrasound device with the following parameters: 0.05 W/cm2 with 10% duty cycle, frequency of 3 MHz and 8 pulses.
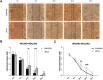
Results: At the parameters used, the ultrasound did not directly affect bCSC proliferation, with no evident changes in morphology. In contrast, the ultrasound protocol affected the migration and invasion ability of bCSCs, limiting their capacity to advance while a major affection was detected on their angiogenic properties. LIPUS-treated bCSCs were unable to transform into aggressive metastatic cancer cells, by decreasing their migration and invasion capacity as well as vessel formation. Finally, RNA-seq analysis revealed major changes in gene expression, with 676 differentially expressed genes after LIPUS stimulation, 578 upregulated and 98 downregulated.
Conclusions: Overall, these results highlight the potential of LIPUS as a promising non-invasive therapy to target bCSCs and attenuate its capacity to drive migration, invasion, angiogenesis and, ultimately, tumor malignancy. Besides, the ability of LIPUS to modulate gene expression points out its capacity to broadly influence the cellular transcriptome. Therefore, the application of LIPUS as an antitumor therapeutic agent targeting bCSCs may offer a promising new approach to treat cancer. In vivo functional experiments will determine in the future the relevance of LIPUS application for the development of metastasis.
Keywords: Angiogenesis; Breast cancer stem cell; Invasion; Low-intensity pulsed ultrasound; Migration.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures




Similar articles
-
Application of low-intensity pulsed therapeutic ultrasound on mesenchymal precursors does not affect their cell properties.PLoS One. 2021 Feb 11;16(2):e0246261. doi: 10.1371/journal.pone.0246261. eCollection 2021. PLoS One. 2021. PMID: 33571276 Free PMC article.
-
Attenuation of orthodontically induced inflammatory root resorption by using low-intensity pulsed ultrasound as a therapeutic modality- a systematic review.BMC Oral Health. 2024 Jan 10;24(1):67. doi: 10.1186/s12903-023-03741-1. BMC Oral Health. 2024. PMID: 38200481 Free PMC article.
-
Low-intensity pulsed ultrasound affects proliferation and migration of human hepatocellular carcinoma cells.J Cancer Res Clin Oncol. 2025 Apr 10;151(4):136. doi: 10.1007/s00432-025-06183-0. J Cancer Res Clin Oncol. 2025. PMID: 40208346 Free PMC article.
-
Low-Intensity Pulsed Ultrasound Stimulates Osteogenic Differentiation of Periosteal Cells In Vitro.Tissue Eng Part A. 2021 Jan;27(1-2):63-73. doi: 10.1089/ten.TEA.2019.0331. Epub 2020 May 21. Tissue Eng Part A. 2021. PMID: 32164486
-
Jingle Cell Rock: Steering Cellular Activity With Low-Intensity Pulsed Ultrasound (LIPUS) to Engineer Functional Tissues in Regenerative Medicine.Ultrasound Med Biol. 2024 Dec;50(12):1973-1986. doi: 10.1016/j.ultrasmedbio.2024.08.016. Epub 2024 Sep 16. Ultrasound Med Biol. 2024. PMID: 39289118 Review.
References
-
- Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229–63. - PubMed
-
- Liang Y, Zhang H, Song X, Yang Q. Metastatic heterogeneity of breast cancer: Molecular mechanism and potential therapeutic targets. Semin. Cancer Biol. Academic Press; 2020. pp. 14–27. - PubMed
-
- de Visser KE, Joyce JA. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell. Cell Press; 2023. pp. 374–403. - PubMed
-
- Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–63. - PubMed
LinkOut - more resources
Full Text Sources

