Pharmacokinetic Properties and Therapeutic Effectiveness of Remimazolam in ICU Patients With Mechanical Ventilation: A Preliminary Study
- PMID: 40517312
- PMCID: PMC12167508
- DOI: 10.1002/prp2.70130
Pharmacokinetic Properties and Therapeutic Effectiveness of Remimazolam in ICU Patients With Mechanical Ventilation: A Preliminary Study
Abstract
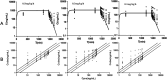
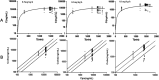
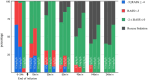
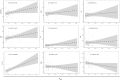
The pharmacokinetic (PK) profile of remimazolam, a ultra-short-acting benzodiazepine, has been investigated for procedural sedation and anesthesia, but its pharmacokinetics, pharmacodynamics, and optimal dosing for ICU sedation are still unclear. This prospective, single-center, double-blind randomized controlled trial studied ICU adults on mechanical ventilation for over 24 h. Participants were divided into three groups, each receiving a 0.2 mg/kg remimazolam loading dose in less than a minute, followed by maintenance doses of 0.1, 0.3, or 0.5 mg/kg/h. Plasma concentrations of remimazolam and its metabolites were measured using UPLC-MS/MS, and pharmacokinetic parameters were calculated using one-compartmental methods with WinNolin. The study also assessed pharmacodynamic indicators (RASS score) and the impact of the clinical indicators on pharmacokinetic parameters. The study on 36 ICU patents using a one-compartment model found that after 24 h of continuous intravenous remimazolam infusion, the drug had a median clearance rate of 22.23 mL/kg/min and a volume of distribution of 2656.58 mL/kg. The half-life was 101.791 min in ventilated patients, while its metabolites had a slower clearance rate of 0.49 mL/kg/min and an longer half-life of 656.02 min. Sedation levels were mild to moderate at dosed of 0.1-0.3 mg/kg/h. Liver function significantly affected remimazolam metabolism, influencing the half-life (R2 = 0.36, p = 0.00013) and clearance (R2 = 0.13, p = 0.04). The pharmacokinetic study indicates that remimazolam is effective and safe for ICU patients on mechanical ventilation, with a 24-h infusion demonstrating rapid clarence and a clear dose-effect relationship. It provides mild to moderate sedation at 0.1-0.3 mg/kg/h, but caution is advised for patients with severe liver dysfunction due to its impact on drug metabolism. Trial Registration: ClinicalTrials.gov identifier: NCT05480787.
Keywords: intensive care; mechanical ventilation; pharmacokinetics; remimazolam; sedation.
© 2025 The Author(s). Pharmacology Research & Perspectives published by British Pharmacological Society and American Society for Pharmacology and Experimental Therapeutics and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Remimazolam Besylate Versus Propofol for Short-Term Sedation in Critically Ill Patients Receiving Mechanical Ventilation: Protocol for a Multicenter Randomized Non-inferior Trial.Adv Ther. 2025 Aug;42(8):4081-4088. doi: 10.1007/s12325-025-03268-7. Epub 2025 Jun 10. Adv Ther. 2025. PMID: 40493335
-
Efficacy and safety of remimazolam combined with remifentanil for sedation during awake fiberoptic intubation: a randomized controlled trial.Ann Med. 2025 Dec;57(1):2527951. doi: 10.1080/07853890.2025.2527951. Epub 2025 Jul 3. Ann Med. 2025. PMID: 40605581 Free PMC article. Clinical Trial.
-
Pharmacokinetics and Pharmacodynamics of Remimazolam for Procedural Sedation in Children and Adolescents.Anesthesiology. 2025 Aug 1;143(2):368-382. doi: 10.1097/ALN.0000000000005560. Epub 2025 May 12. Anesthesiology. 2025. PMID: 40355106 Free PMC article. Clinical Trial.
-
Corticosteroids for the treatment of Duchenne muscular dystrophy.Cochrane Database Syst Rev. 2016 May 5;2016(5):CD003725. doi: 10.1002/14651858.CD003725.pub4. Cochrane Database Syst Rev. 2016. PMID: 27149418 Free PMC article.
-
Sertindole for schizophrenia.Cochrane Database Syst Rev. 2005 Jul 20;2005(3):CD001715. doi: 10.1002/14651858.CD001715.pub2. Cochrane Database Syst Rev. 2005. PMID: 16034864 Free PMC article.
References
-
- Devlin J. W., Skrobik Y., Gélinas C., et al., “Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU,” Critical Care Medicine 46, no. 9 (2018): e825–e873, 10.1097/ccm.0000000000003299. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

