Progressive Response of Repeated Treatment with High-Concentration (179 mg) Capsaicin Patch in Peripheral Neuropathic Pain After Surgical or Traumatic Nerve Injury: Findings from the 12-Month German CASPAR Registry Study
- PMID: 40517340
- PMCID: PMC12279645
- DOI: 10.1007/s40122-025-00752-4
Progressive Response of Repeated Treatment with High-Concentration (179 mg) Capsaicin Patch in Peripheral Neuropathic Pain After Surgical or Traumatic Nerve Injury: Findings from the 12-Month German CASPAR Registry Study
Abstract
Introduction: Peripheral neuropathic pain after nerve injury (PNI) caused by surgery or trauma can severely impact daily life. The high-concentration capsaicin patch (HCCP, 179 mg) is a topical therapy approved for peripheral neuropathic pain, including PNI. This study utilizes data from the German Pain e-Registry (GPeR) to investigate the real-world effectiveness of HCCP in managing PNI across multiple treatments over 1 year.
Methods: CASPAR is a retrospective, non-interventional cohort study investigating patients with peripheral neuropathic pain treated with HCCP. The present analysis included 499 patients with PNI who received ≥ 1 HCCP with ≥ 12 months of follow-up. Key measures included pain intensity, quality of life (QoL), affective distress, sleep disturbances, and overall functioning. Furthermore, analgesic use and adverse events associated with HCCP treatment were evaluated.
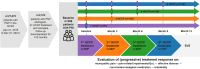
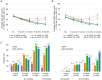
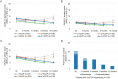
Results: The mean average daily pain intensity (API) decreased from 52.5 mm on the visual analog scale (VAS) at baseline to 21.5 mm at month 12 in patients receiving four HCCPs. At month 12, a ≥ 30% reduction in API was observed in 25.8%, 44.9%, 85.3%, and 97.8% of patients receiving one, two, three, and four HCCP treatments, respectively. Significant improvements were also noted in physical and mental QoL, sleep, mood, and daily functioning. Patients receiving three or four HCCP treatments maintained pain relief and symptom improvements over the 12-month period, whereas those who discontinued treatment after one or two treatments experienced symptom rebound. In addition, repeated HCCP treatments were associated with a marked reduction in concomitant analgesic use and an increase in days of normal activities. Adverse events were mild-to-moderate application-site reactions, consistent with the well-established safety profile of HCCP.
Conclusions: HCCP treatment is associated with reductions in pain intensity and improvements in sleep, mood, and overall QoL in patients with PNI. These benefits are amplified with continued treatment and are accompanied by reduced use of concomitant analgesics and more days of usual activities, although a direct causal relationship cannot be confirmed within the context of this observational study.
Clinical trial registration: EU PAS number: EUPAS1000000106.
Keywords: High-concentration capsaicin patch; Peripheral nerve injury; Peripheral neuropathic pain; Real-word data; Topical treatment.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflicts of Interest: Michael A. Überall is the director of the private Institute for Neurosciences and the Center of Excellence for Health Services Research in Nuremberg, honorary vice president of the German Pain Association, and president of the German Pain League. In the past 5 years, Michael A. Überall has received lecture and consulting fees as well as expense reimbursements for associated travel activities from Grünenthal. Christian Simanski: During the last 5 years, Christian Simanski received speaker and consultancy fees from Grünenthal. A professor at the Chair of Orthopedics and Trauma Surgery at the private University of Witten/Herdecke, Campus Cologne-Merheim. Took part in expert and consulting activities for the German Statutory Accident Insurance (DGUV) and all affiliated professional associations. Took part in expert activity for the District Court of Essen and Cologne. Mike Zellnig: Fees and reimbursement of travel and congress costs in the last 3 years received from Grünenthal, Deutsche Schmerzgesellschaft, BG RCI, VBG, BGW, BG Bau, BG Verkehr, BGHW, Hochschule der Gesetzlichen Unfallversicherung, Hochschule Bonn-Rhein-Sieg, and HDI Versicherung. Mariëlle Eerdekens, Sylvia Engelen, Myriam Heine, Fabienne Percot, Rita Freitas, Lucia Garcia Guerra, and Tamara Quandel are employees of Grünenthal GmbH, Germany, or its affiliates. Ethical Approval: This retrospective, non-interventional study used fully anonymized data from the GPeR registry. Ethical committee approval was not required, as no identifiable information was analyzed, and no study-specific interventions took place. The study concept and data use were endorsed by the steering committees of the German Pain Association and the German Pain League. Permission to use the data was granted to O.Meany-MDPM GmbH under formal contractual agreement, and all participating physicians and patients had provided prior written informed consent.
Figures


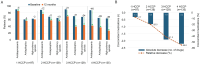
Similar articles
-
Progressive improvements in patient-reported outcomes with the high-concentration capsaicin patch: A retrospective cohort study in patients with painful diabetic peripheral neuropathy (CASPAR study).J Diabetes Complications. 2025 Sep;39(9):109085. doi: 10.1016/j.jdiacomp.2025.109085. Epub 2025 May 22. J Diabetes Complications. 2025. PMID: 40499399
-
Topical capsaicin (high concentration) for chronic neuropathic pain in adults.Cochrane Database Syst Rev. 2017 Jan 13;1(1):CD007393. doi: 10.1002/14651858.CD007393.pub4. Cochrane Database Syst Rev. 2017. PMID: 28085183 Free PMC article.
-
Topical capsaicin (high concentration) for chronic neuropathic pain in adults.Cochrane Database Syst Rev. 2013 Feb 28;(2):CD007393. doi: 10.1002/14651858.CD007393.pub3. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2017 Jan 13;1:CD007393. doi: 10.1002/14651858.CD007393.pub4. PMID: 23450576 Updated.
-
Acupuncture for neuropathic pain in adults.Cochrane Database Syst Rev. 2017 Dec 2;12(12):CD012057. doi: 10.1002/14651858.CD012057.pub2. Cochrane Database Syst Rev. 2017. PMID: 29197180 Free PMC article.
-
Tramadol for neuropathic pain in adults.Cochrane Database Syst Rev. 2017 Jun 15;6(6):CD003726. doi: 10.1002/14651858.CD003726.pub4. Cochrane Database Syst Rev. 2017. PMID: 28616956 Free PMC article.
References
-
- Schug SA, Lavand’homme P, Barke A, et al. The IASP classification of chronic pain for ICD-11: chronic postsurgical or posttraumatic pain. Pain. 2019;160(1):45–52. - PubMed
-
- Duale C, Ouchchane L, Schoeffler P, Group EI, Dubray C. Neuropathic aspects of persistent postsurgical pain: a French multicenter survey with a 6-month prospective follow-up. J Pain. 2014;15(1):24.e1-e0. - PubMed
-
- Fletcher D, Stamer UM, Pogatzki-Zahn E, et al. Chronic postsurgical pain in Europe: an observational study. Eur J Anaesthesiol. 2015;32(10):725–34. - PubMed
-
- Haroutiunian S, Nikolajsen L, Finnerup NB, Jensen TS. The neuropathic component in persistent postsurgical pain: a systematic literature review. Pain. 2013;154(1):95–102. - PubMed
-
- Simanski CJ, Althaus A, Hoederath S, et al. Incidence of chronic postsurgical pain (CPSP) after general surgery. Pain Med. 2014;15(7):1222–9. - PubMed
LinkOut - more resources
Full Text Sources

