Efficacy and Safety of Once-Monthly Paliperidone Palmitate Long-Acting Injections in Chinese Patients with Early-, Mid-, and Late-Phase Schizophrenia: A Post-Hoc Analysis of Three Phase 4 Studies
- PMID: 40517352
- PMCID: PMC12263469
- DOI: 10.1007/s40263-025-01194-4
Efficacy and Safety of Once-Monthly Paliperidone Palmitate Long-Acting Injections in Chinese Patients with Early-, Mid-, and Late-Phase Schizophrenia: A Post-Hoc Analysis of Three Phase 4 Studies
Abstract
Background: Long-acting injectable (LAI) antipsychotics have improved treatment adherence and continuity compared with oral antipsychotics. However, evidence gaps persist in the endorsement of LAIs for early-phase schizophrenia in China. This post-hoc analysis evaluated the efficacy and safety of once-monthly paliperidone palmitate (PP1M), an LAI antipsychotic, following early-, mid-, and late-phase use in Chinese patients with schizophrenia.
Methods: Data from three phase 4 studies (NCT01527305, NCT01947803, and NCT01685931) were used. Chinese patients with schizophrenia (disease duration early: ≤ 2 years; mid: > 2 to ≤ 5 years; late: > 5 years) who received 13 weeks of PP1M treatment were included. The primary endpoint was change in Positive and Negative Syndrome Scale (PANSS) total score from baseline to week 13, and the secondary and exploratory endpoints included change in Clinical Global Impression of Severity scale (CGI-S), PANSS responder rate relative to baseline PANSS total scores (≤ 70, 70-90 [exclusive], and ≥ 90), and treatment-emergent adverse events (TEAEs).
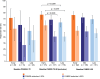
Results: In total, 1053 patients (early: 383; mid: 290; late: 380) were included. Following PP1M, improvements in efficacy outcomes were observed in all phases of schizophrenia including PANSS total score from baseline to week 13 (least square [LS]-mean change early: - 31.6; mid: - 28.4; late: - 25.6; p = 0.0003 across three groups) and CGI-S (median reduction early: - 2.0; mid: - 1.0; late: - 1.0). The greatest improvements in efficacy outcomes were consistently seen in early-phase patients, indicated by differences in PANSS total score (baseline to week 13 LS-mean differences mid versus early: 3.2 [p = 0.2175], late versus mid: 2.8 [p = 0.2783], and late versus early: 6.0 [p = 0.0011]), and nominal significant differences in CGI-S (mid versus early: p = 0.0015 and late versus early: p = 0.0180). Earlier administration of PP1M results in greater efficacy outcomes regardless of the initial disease severity. For patients with a PANSS score ≤ 70 at baseline, reductions of ≥ 30% were observed in 71.4%, 60.0%, and 50.0% (p = 0.0003 across three groups), and for patients with a PANSS score ≥ 90 at baseline, reductions of ≥ 30% were observed in 76.4%, 72.5%, and 69.6% (p = 0.0003 across three groups) of patients in the early-, mid-, and late-phase, respectively. The proportion of patients experiencing ≥ 1 TEAE (early: 44.3%; mid: 38.4%; late: 39.8%) was numerically higher in the early phase. Incidences of serious TEAEs (early: 2.8%; mid: 4.0%; late: 4.2%) and TEAEs leading to death (early: 0.3%; mid: 0.0%; late: 1.6%) were observed.
Conclusions: Treatment with PP1M improves efficacy outcomes in Chinese adult patients with schizophrenia. However, when PP1M was used in earlier phases of schizophrenia, consistently greater improvements in efficacy outcomes were observed compared with later phases, regardless of disease severity at baseline. Safety data were consistent with the existing profile for PP1M. These findings support the use of PP1M in Chinese patients with early-phase schizophrenia.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Funding: This work was funded by Xian Janssen Pharmaceutical Co., Ltd. Conflict of interest: Qian Li and Tianmei Si declare that they have no conflicts of interest. Yang Li, Chong Ye, and Miaomiao Jia are employees of Xian Janssen Pharmaceutical Co., Ltd, which is a subsidiary of Johnson & Johnson. Jianmin Zhuo is an employee of Johnson & Johnson (China) Investment Ltd. Yishen Yang is an employee of IQVIA, which was contracted by Johnson & Johnson for the presented analyses. Data availability statement: Underlying data supporting this study are not publicly available due to ethical reasons. Ethics approval: For each study (NCT01527305, NCT01947803, and NCT01685931), the protocols were reviewed and approved by the local study site’s institutional review board or by an independent ethics committee. The studies were conducted in accordance with the Declaration of Helsinki and Good Clinical Practice and any applicable local regulatory requirements. Consent to participate: Written informed consent was obtained from the patients prior to entering the phase 4 studies. Consent to publish: Not applicable. Code availability: Not applicable. Author contributions: Qian Li, Yang Li, and Tianmei Si conceived the idea and design of the study. Yishen Yang and Jianmin Zhuo performed data collection, extraction, and analysis. Qian Li, Yang Li, Chong Ye, Jianmin Zhuo, Yishen Yang, and Miaomiao Jia drafted the paper. Tianmei Si provided clinical support for drafting the paper. All authors reviewed the final manuscript. All authors have read and approved the final submitted manuscript and agree to be accountable for the work.
Figures

References
-
- WHO. Schizophrenia. https://www.who.int/news-room/fact-sheets/detail/schizophrenia. Accessed 20 Sept 2024.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

