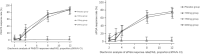Investigation of the Efficacy and Safety of Xeligekimab (GR1501) in Patients with Moderate-to-Severe Plaque Psoriasis: A Multicenter, Randomized, Double-Blind Phase II Clinical trial
- PMID: 40517362
- PMCID: PMC12454832
- DOI: 10.1007/s13555-025-01450-x
Investigation of the Efficacy and Safety of Xeligekimab (GR1501) in Patients with Moderate-to-Severe Plaque Psoriasis: A Multicenter, Randomized, Double-Blind Phase II Clinical trial
Erratum in
-
Correction: Investigation of the Efficacy and Safety of Xeligekimab (GR1501) in Patients with Moderate-to-Severe Plaque Psoriasis: A Multicenter, Randomized, Double-Blind Phase II Clinical trial.Dermatol Ther (Heidelb). 2025 Oct;15(10):2817-2818. doi: 10.1007/s13555-025-01480-5. Dermatol Ther (Heidelb). 2025. PMID: 40705212 Free PMC article. No abstract available.
Abstract
Introduction: Psoriasis is a chronic inflammatory skin disease. This study evaluated the efficacy and safety of xeligekimab (GR1501), a novel anti-interleukin-17A (anti-IL-17A) monoclonal antibody, in Chinese patients with moderate-to-severe plaque psoriasis.
Methods: In this multicenter, randomized, double-blind, phase II trial, 199 patients were assigned (1:1:1:1) to receive placebo (n = 49) or xeligekimab 100 mg (n = 50), 150 mg (n = 49), or 200 mg (n = 51) every 4 weeks for 12 weeks. All participants then entered a 40-week extension receiving xeligekimab 200 mg every 4 or 8 weeks. The primary endpoint was a 75% reduction in the Psoriasis Area and Severity Index (PASI 75) at week 12. Secondary endpoints included PASI 75, PASI 90 (≥ 90% improvement), and a static Physician's Global Assessment (sPGA) score of 0/1 (clear/almost clear) at week 52. Safety, pharmacokinetics (PK), and anti-drug antibodies (ADA) were also assessed.
Results: At week 12, PASI 75 response rates for the 100, 150, and 200 mg groups were 86.0%, 89.8%, and 88.2%, respectively, versus 2.0% for placebo (P < 0.05). At week 52, PASI 75, PASI 90, and sPGA 0/1 response rates remained high in both 4-week (98.8%, 83.3%, 77.4%) and 8-week (92.9%, 83.3%, 78.6%) groups. No dose-dependent safety issues or ADA positivity were observed.
Conclusion: Xeligekimab demonstrated strong efficacy, sustained response, and favorable safety in patients with moderate-to-severe plaque psoriasis.
Trial registration number: ChiCTR1800017956.
Keywords: Drug-related side effects and adverse reactions; Immunity; Psoriasis; Randomized controlled trials; Xeligekimab.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: Lin Cai, Chengxin Li, Shanshan Li, Xiaodong Zhang, Gang Wang, Jianbin Yu, Kun Huang, Hong Fang, Yangfeng Ding, Jinyan Wang, Congjun Jiang, Qianjin Lu, Juan Tao, and Jianzhong Zhang declare that they have no competing interests. Ethical Approval: The ethics committees of each study center reviewed the study protocol and informed consent requirements (2020PHA072-001, GR1501-004). The study was conducted according to the ethical principles of the Declaration of Helsinki. Written informed consent was obtained from each patient before randomization. The trial registered with the number of ChiCTR1800017956.
Figures
References
-
- Yu H, Heng G, Chen K. Disease burden and unmet medical needs in Chinese patients with moderate-to-severe psoriasis: a systematic review. Chin J Dermatol. 2023;56(10):965–72.
LinkOut - more resources
Full Text Sources
Research Materials