Modulation of motor excitability reflects traveling waves of neural oscillations
- PMID: 40517384
- PMCID: PMC12257788
- DOI: 10.1016/j.celrep.2025.115864
Modulation of motor excitability reflects traveling waves of neural oscillations
Abstract
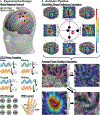
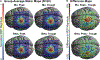
Neural traveling waves represent an important endogenous phenomenon with structural and functional relevance in the human brain. These waves, commonly recorded via electroencephalogram (EEG) or electrocorticography (ECoG), are implicated in a range of brain processes. However, it remains unclear how they influence neural excitability across brain regions. Advancements in real-time control of brain stimulation present opportunities to compare traveling waves and excitation. Here, we investigate how sensorimotor mu (8-13 Hz) and beta (14-30 Hz) traveling waves affect motor cortex excitability using real-time EEG-controlled transcranial magnetic stimulation (TMS). We observed gradients in the mediolateral direction and then validated these findings using ECoG recordings in a human participant and a nonhuman primate. Our results demonstrate that neuronal excitability reflects the natural patterns of sensorimotor traveling waves. This provides important evidence of traveling waves modulating neural excitability in humans. This opens possibilities for more effective stimulation protocols aligned with intrinsic brain dynamics.
Keywords: CP: Neuroscience; TMS-EEG; closed-loop; electrocorticography; electroencephalography; motor cortex; real-time stimulation; transcranial magnetic stimulation; traveling waves.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests A.O., I.A., and S.S. are inventors on patent applications describing TMS closed-loop stimulation technology.
Figures


Similar articles
-
Sensory Entrained TMS (seTMS) Enhances Motor Cortex Excitability.Hum Brain Mapp. 2025 Jul;46(10):e70267. doi: 10.1002/hbm.70267. Hum Brain Mapp. 2025. PMID: 40622189 Free PMC article.
-
Stroke Recovery-Related Changes in Cortical Reactivity Based on Modulation of Intracortical Inhibition.Stroke. 2024 Jun;55(6):1629-1640. doi: 10.1161/STROKEAHA.123.045174. Epub 2024 Apr 19. Stroke. 2024. PMID: 38639087
-
Involvement of muscarinic acetylcholine receptor-mediated cholinergic neurotransmission in TMS-EEG responses.Prog Neuropsychopharmacol Biol Psychiatry. 2025 Jan 10;136:111167. doi: 10.1016/j.pnpbp.2024.111167. Epub 2024 Oct 9. Prog Neuropsychopharmacol Biol Psychiatry. 2025. PMID: 39383933 Clinical Trial.
-
Effects of transcranial magnetic stimulation in modulating cortical excitability in patients with stroke: a systematic review and meta-analysis.J Neuroeng Rehabil. 2022 Feb 22;19(1):24. doi: 10.1186/s12984-022-00999-4. J Neuroeng Rehabil. 2022. PMID: 35193624 Free PMC article.
-
The number of stimuli required to reliably assess corticomotor excitability and primary motor cortical representations using transcranial magnetic stimulation (TMS): a systematic review and meta-analysis.Syst Rev. 2017 Mar 6;6(1):48. doi: 10.1186/s13643-017-0440-8. Syst Rev. 2017. PMID: 28264713 Free PMC article.
References
-
- Ermentrout GB, and Kleinfeld D (2001). Viewpoint Traveling Electrical Waves in Cortex: Insights from Phase Dynamics and Speculation on a Computational Role the Results The Amplitude of the Wave Is a Periodic Function of Time. Soc. Neurosci. 29, 33–44. - PubMed
-
- Hughes JR (1995). The Phenomenon of Travelling Waves: A Review. Clin. Electroencephalogr. 26, 1–6. - PubMed
-
- Hindriks R, van Putten MJAM, and Deco G (2014). Intra-cortical propagation of EEG alpha oscillations. Neuroimage 103, 444–453. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

