Outcomes of haploidentical vs mismatched unrelated donor HCT with posttransplant cyclophosphamide prophylaxis
- PMID: 40517414
- PMCID: PMC12345264
- DOI: 10.1182/bloodadvances.2025016236
Outcomes of haploidentical vs mismatched unrelated donor HCT with posttransplant cyclophosphamide prophylaxis
Abstract
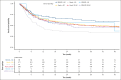
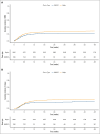
Limited data exist comparing haploidentical and mismatched unrelated donor (MMUD) hematopoietic cell transplantation (HCT) with posttransplantation cyclophosphamide for graft-versus-host disease prophylaxis, especially considering donor age. Herein, we report the outcomes of 660 haploidentical and 195 MMUD HCT recipients treated at MD Anderson Cancer Center. Beyond standard Cox proportional hazards modeling, we used inverse probability of treatment weighting (IPTW) and matched-pair analysis, and performed additional analysis by incorporating an external MMUD validation cohort from the Center for International Blood and Marrow Transplant Research (CIBMTR). The primary outcome was overall survival (OS). In multivariable analysis, haploidentical donors had a hazard ratio (HR) of 1.20 (95% confidence interval [CI], 0.93-1.54; P = .16) compared with the MMUD group. Donor age showed a nonlinear association with OS. These findings were corroborated by IPTW, matched-pair analyses, and CIBMTR validation analyses. Exploratory analysis revealed inferior OS for older (age of >50 years) haploidentical donor group compared with younger (age of <30 years) MMUD recipients (HR, 1.91; 95% CI, 1.21-3.01; P = .005). Our analyses suggest that although donor type may play a role, there was a more prominent role for donor age in influencing OS. Moreover, our findings indicate a potential nuance wherein the impact of donor type may vary by donor age. Further research, particularly with larger cohorts, is needed to fully elucidate the complex and potentially interacting roles of donor type and donor age, along with HLA factors.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: K.R. and The University of Texas MD Anderson Cancer Center have an institutional financial conflict of interest with Takeda Pharmaceutical and Affimed GmbH. K.R. participates on the scientific advisory board for Avenge Bio, Virogin Biotech, Navan Technologies, Caribou Biosciences, Bit Bio Limited, Replay Holdings, oNKo-innate, the Alliance for Cancer Gene Therapy, Innate Pharma, and Shinobi Therapeutics; and is the scientific founder of Syena. The remaining authors declare no competing financial interests.
Figures



References
-
- Cusatis R, Litovich C, Feng Z, et al. Current trends and outcomes in cellular therapy activity in the United States, including prospective patient-reported outcomes data collection in the Center for International Blood and Marrow Transplant Research Registry. Transpl Cell Ther. 2024;30(9):917.e1–917.e12. - PMC - PubMed
-
- Verneris MR, Lee SJ, Ahn KW, et al. HLA mismatch is associated with worse outcomes after unrelated donor reduced-intensity conditioning hematopoietic cell transplantation: an analysis from the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transpl. 2015;21(10):1783–1789. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

